Most reactions proceed faster at higher temperatures, and many industrial processes are carried out at high temperatures.
Question:
Most reactions proceed faster at higher temperatures, and many industrial processes are carried out at high temperatures. However, for exothermic reactions increasing the temperature reduces the equilibrium constant and thus the reaction yield. Suppose you are studying how to increase the yield of the ammonia synthesis reaction and decide to investigate the effect of raising the temperature. The equilibrium constant K for the synthesis of ammonia (reaction A) is 6.8 * 105 at 298 K. Predict its value at 400. K.
ANTICIPATE The synthesis of ammonia is known to be exothermic, so you should expect the equilibrium constant to be smaller at the higher temperature.
PLAN To use the van ’t Hoff equation, you need the standard reaction enthalpy, which can be calculated from the standard enthalpies of formation in Appendix 2A. Equation 1 requires you to use the “molar” convention.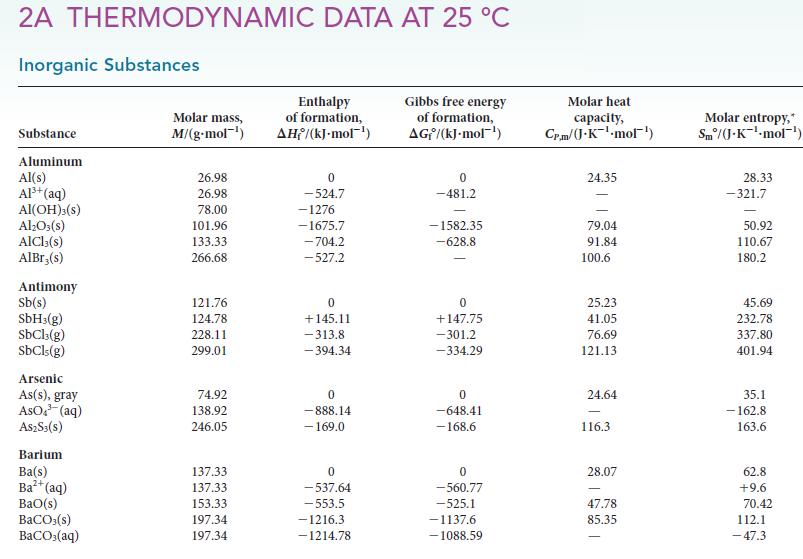
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





