State what happens to the concentration of the indicated substance when the total pressure on each of
Question:
State what happens to the concentration of the indicated substance when the total pressure on each of the following equilibria is increased (by compression):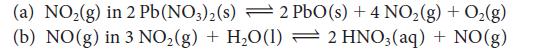
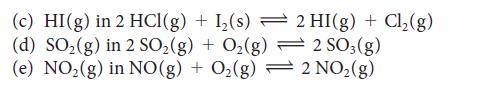
Transcribed Image Text:
(a) NO₂(g) in 2 Pb (NO3)2(s) 2 PbO (s) + 4 NO₂ (g) + O₂(g) 2 HNO3(aq) + NO(g) (b) NO(g) in 3 NO₂(g) + H₂O(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a In the reaction 2 PbNO32s 2 PbOs 4 NO2g O2g increasing the total pressure by compression will shif...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
When we double the compression ratio of an ideal Otto cycle, what happens to the maximum gas temperature and pressure when the state of the air at the beginning of the compression and the amount of...
-
Consider the equilibrium CO(g) + H 2 O(g) CO 2 (g) + H 2 (g). (a) If the partial pressure of CO 2 is increased, what happens to the partial pressure of H 2 ? (b) If the partial pressure of CO is...
-
Each of the following three data sets represents the IQ scores of a random sample of adults. IQ scores are known to have a mean and median of 100. For each data set, determine the sample standard...
-
Why is the number of equivalent units for materials only sometimes equal to the equivalent units for conversion?
-
A hydrocarbon stream in a petroleum refinery is to be separated at 1,500 kPa into two products under the conditions shown below. Using the data given, compute the minimum work of separation, Wmin ,...
-
Jenny Lanstrom regularly visits her grandfather, Mike Lanstrom, every Thursday night. Jennys grandfather has been a widower for the past six years. Jennys grandfather is very intelligent. He is a...
-
Verify that the \(j\)-factor is related to the drag coefficient by the relation \[j_{\mathrm{D}}=\frac{c_{\mathrm{D}}}{2}\] for mass transfer for flow over a flat plate.
-
The trial balance of Harper, Inc., at September 30, 2016, does not balance: The accounting records hold the following errors: a. Recorded a $500 cash revenue transaction by debiting Accounts...
-
Your company needs fresh cash (10 Mio ). In the board meeting the CFO was proposing 10,000 bonds with yield to maturity of 100 % for a) 1 or b)2 years with face values of 1,000 and coupons of i) 10...
-
Obtain the general solution of the following differential equations: (a) (D 2D + 5)y = 0 (b) (D6 + 9D* + 24D + 16)y = 0
-
Two unknown compounds were being studied. Compound C is molecular and compound D is an ionic compound known to dissociate into ions completely in dilute aqueous solutions. A solution containing 0.30...
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl(g), K = 1.8 10- at 500 K CaO(s) + CO(g), K = 167 at 1073 K
-
A recent study analyzed the influence of looks on a candidates chances of getting called for an interview. Interestingly, more interviews were granted to plain looking women than to attractive women....
-
Compare Statement 1 and Statement 2 and identify which best explains the view of a speculative volatility trader and which best explains the view of a hedger of volatility. Justify your response....
-
In Nowackis backtesting of the factor-based strategy for the new fund, the calculated information coefficient should be based on: A. FS(t) and SR(t). B. FS(t) and SR(t 1). C. SR(t) and FS(t 1)....
-
The most appropriate trading strategy for the sell order of Music Plus shares is: A. trading in the open market. B. selling at the closing auction for the day. C. passive trading over the course of...
-
To fill the remaining portion of the ABC order, Yellow is using: A. an arrival price trading strategy. B. a TWAP participation strategy. C. a VWAP participation strategy. Robert Harding is a...
-
Recommend a solution that will provide the fund manager the opportunity to earn currency alpha through active foreign exchange management. Gupta and the fund manager of Portfolio A discuss the...
-
A construction company has an estimated profit, before taxes, of $547,852 for the year. Included in the companys costs is $65,258 for meals and entertainment. Determine the taxable income for the...
-
Troy is a qualified radiologist who operates a successful radiology practice from purpose- built rooms attached to his house. Troy works in the practice three days a week, and the other two days he...
-
A 1.25 mole sample of an ideal gas is expanded from 320. K and an initial pressure of 3.10 bar to a final pressure of 1.00 bar, and C P,m = 5/2R. Calculate w for the following two cases: a. The...
-
What reagents would you use to perform each of the following transformations? a. b. H.
-
Propose a mechanism for the following reaction. NaH Br
-
For the point P(-6,-13) and Q(1, -8), find the distance d(P,Q) and the: coordinates of the midpoint M of the segment PQ. What is the distance? 74 (Simplify your answer. Type an exact answer, using...
-
Show that if two real symmetric matrices A, B are congruent then there is a linear change of variables to x1, x such that q(x1,...,xn) = qB(x,,xn).
-
When the polynomial f(x) = 2x- 17x + 15 is divided by g(x) = (x-1), the result is of the form ax+ bx-c, where a, b and c EN. The values of a, b and c are Do not enter spaces or commas.) (Enter digits...

Study smarter with the SolutionInn App


