Evaluate K c for each of the following equilibria from the value of K: (a) 2 NOCI(g)
Question:
Evaluate Kc for each of the following equilibria from the value of K: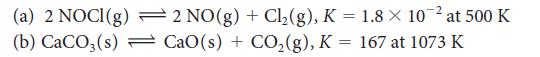
Transcribed Image Text:
(a) 2 NOCI(g) (b) CaCO3(s) 2 NO(g) + Cl₂(g), K = 1.8 × 10-² at 500 K CaO(s) + CO₂(g), K = 167 at 1073 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a K c...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Evaluate K c for each of the following equilibria from the value of K: (a) 2 SO(g) + O(g) 2 SO3(g), K = 3.4 at 1000. K (b) NHHS(s) - NH3(g) + HS(g), K = 9.4 x 10- at 24 C
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N203(g)NO2(8) +NO(g 2NO(g)+O2(g) 2NO2(g) PC13(g) + 3NH3(g)-P(NH2)3(g) + 3HC(g) c. d.
-
Write equilibrium-constant expressions Kc for each of the following reactions. a. N2(g) 2H2g) N2H4(g) b. 2NOCI(g) 2N0(g) + Cl2(g) c. 2NO(g) 2H2(g)N2(8) 2H20(g)
-
Which of the following variables was controlled in Experiment 1? F. Amount of yeast G. Percent of molasses H. Percent of sucrose J. Carbon dioxide levels Experiment 1 Since yeast needs sucrose to...
-
Explain why the separation of a stream containing 10 wt% acetic acid in water might be more economical by liquid-liquid extraction with ethyl acetate than by distillation.
-
In what ways do e-business transactions pose heightened fraud risks?
-
The factor \(j_{\mathrm{D}}\) is also used in the correlation. This is defined as \[j_{\mathrm{D}}=S t(S c)^{2 / 3}=\frac{k_{\mathrm{m}}}{v_{\text {ref }}}(S c)^{2 / 3}\] where \(S t\) is the Stanton...
-
Use the following information from separate companies a through f to compute times interest earned. Which company indicates the strongest ability to pay interest expense as it comes due? (Round...
-
Considering pure water at 15.6 C, determine the density and bulk modulus from Table 1.6. From Appendix C, determine the value of standard atmospheric pressure at sea level elevation. Using both...
-
Selected transactions for M. Acosta, an interior decorator, in her first month of business, are as follows. Jan. 2 Invested $10,000 cash in business. 3 Purchased used car for $3,000 cash for use in...
-
State what happens to the concentration of the indicated substance when the total pressure on each of the following equilibria is increased (by compression): (a) NO(g) in 2 Pb (NO3)2(s) 2 PbO (s) + 4...
-
The density of a 5.00% by mass K 3 PO 4 aqueous solution is 1.043 g cm 3 . Determine (a) The molality; (b) The molarity of potassium phosphate in the solution.
-
Vickery Machining Company is nearly finished constructing a specially designed piece of machining equipment when the customer declares bankruptcy and cannot pay for the equipment. Vickery estimates...
-
Explain Guptas statement in light of the strategic choices in currency management available to the portfolio manager. The investment policy statement (IPS) for Portfolio A provides the manager with...
-
The analysis performed by Sardar on the Purity Fund can be best described as being based on: A. a holdings-based approach. B. manager self-identification. C. a returns-based style analysis. Jack...
-
The arrival cost for purchasing the 90,000 shares of BYYP is: A. 164.4 bp. B. 227.2 bp. C. 355.0 bp. Robert Harding is a portfolio manager at ValleyRise, a hedge fund based in the United States....
-
Based upon Deweys chosen investment process for the management of the Purity Fund, rebalancing of the fund will most likely occur: A. at regular intervals. B. in response to changes in...
-
As it relates to the trade policy document, ValleyRise should implement Yellows recommendation related to: A. the list of eligible brokers. B. a policy for the treatment of trade errors. C. a policy...
-
A construction company has an estimated profit, before taxes, of $256,452 for the year. Included in the companys costs is $25,622 for meals and entertainment. Determine the taxable income for the...
-
Synthesize the products by drawing out reagents and intermediates along the way. `N H. OH HO HO
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Calculate S surroundings and S total for part (c) of Problem P5.6. Is the process spontaneous? The state of the surroundings is T = 310.K, P = 0.333 bar.
-
Acid-catalyzed hydration of 1-methylcyclohexene yields two alcohols. The major product does not undergo oxidation, while the minor product will undergo oxidation. Explane.
-
Consider the following linear second order differential equation for y(x). (1-x)y" - 3xy' + 8y = 0 Put the series solution ansatz y(x) = an" into the above differential equation and obtain a...
-
Show that the following quadratic form on R is not positive definite: q(x, y, z) = x + 4xz + 3y+4yz + z.
-
9.1 Let g = C(R). If F is any distribution acting on test functions in the class Co (R), we can define the product of g with F as a distribution; for any $ C(R), (gF, 4) = (F,g). (a) Why was this a...

Study smarter with the SolutionInn App


