State whether reactants or products will be favored by an increase in the total pressure (resulting from
Question:
State whether reactants or products will be favored by an increase in the total pressure (resulting from compression) on each of the following equilibria. If there is no change, explain why that is so.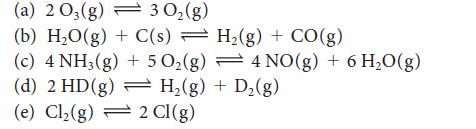
Transcribed Image Text:
(a) 203(g) (b) H₂O(g) + C(s) (c) 4 NH3(g) + 5 O₂(g) (d) 2 HD (g) (e) Cl₂(g) 30₂(g) H₂(g) + CO(g) 2 Cl(g) 4 NO(g) + 6H₂O(g) H₂(g) + D₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Reactants ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
1. In the classical model, it is thought that the long-run: A. and short-run aggregate supply curves are both upward sloping. B. aggregate supply curve is vertical and the short-run aggregate supply...
-
In Problems 5994, solve each inequality. Express your answer using set notation or interval notation. Graph the solution set. 2x2 3 + x
-
The Prism gas permeation process developed by the Monsanto Company is highly selective for hydrogen when using hollow-fiber membranes of materials such as silicone-coated polysulphone. In a typical...
-
Your firm needs a computerized machine tool lathe which costs $40,000 and requires $11,000 in maintenance for each year of its 3-year life. After three years, this machine will be replaced. The...
-
Two rectangular plates of sizes \(L\) and \(W\) are facing each other and separated by a distance \(H\). Derive an expression for the view factor between these disks. Find the limit if \(W\) is...
-
After watching the video on India and Chinas economies, discuss the following: 1. What impact do the political systems of each country have on their efforts toward globalization? 2. How do IP rights...
-
Marketing Analytics 6.1 The Expanding Uses of Data Mining and Analytics in the Restaurant Industry** When Damian Mogavero, a restaurant group CFO, realized the vast need for improved data analytics...
-
Early in its fiscal year ending December 31, 2024, San Antonio Outfitters finalized plans to expand operations. The first stage was completed on March 28 with the purchase of a tract of land on the...
-
Explain the effect that an increase in temperature has on each of the following properties: (a) Viscosity; (b) Surface tension; (c) Vapor pressure; (d) Evaporation rate.
-
Two unknown molecular compounds were being studied. A solution containing 5.00 g of compound A in 100. g of water froze at a lower temperature than a solution containing 5.00 g of compound B in 100....
-
a. From a consideration of the following reactions, calculate Hf for methane, CH4(g). CO2(g) + 2H2(g) HCHO(g) + H2O(g); Ho(kJ) = 35 CO2(g) C(s) + O2(g); Ho(kJ) = 393 HCHO(g) + 2H2(g) CH4(g) +...
-
Identify the new investment approach proposed by Zang for managing the Fund. Justify your response. Bern Zang is the recently hired chief investment officer of the Janson University Endowment...
-
Identify the investment approach currently being used by the Investment Committee for managing the Fund. Justify your response. Bern Zang is the recently hired chief investment officer of the Janson...
-
Fiona Heselwith is a 40-year-old US citizen who has accepted a job with Lyricul, LLC, a UK-based company. Her benefits package includes a retirement savings plan. The company offers both a defined...
-
Dianna Mark is the chief financial officer of Antiliaro, a relatively mature textile production company headquartered in Italy. All of its revenues come from Europe, but the company is losing sales...
-
Describe how each of the following common characteristics of institutional investors supports the Funds allocation to private real estate: i. Scale ii. Investment horizon iii. Governance framework...
-
Using the tax rates for the year 2006, determine the amount of federal income tax that is due for a C corporation that has a taxable income of $356,000.
-
1. Firms may hold financial assets to earn returns. How the firm would classify financial assets? What treatment will such financial assets get in the financial statements in accordance with US GAAP...
-
By looking at the a and b values for the van der Waals equation of state, decide whether 1 mole of O 2 or H 2 O has the higher pressure at the same value of T and V.
-
In the absence of turbulent mixing, the partial pressure of each constituent of air would fall off with height above sea level in the Earths atmosphere as P i = P i 0 e -M,g/RT where P i is the...
-
Propose a mechanism for the following transformation: . 1) Excess LA, 2) H20
-
Find the LCD of the rational expressions in the list. 6 1 t't-1
-
Given the following equation of a line y = = 32 +4, determine the slope of a line that is parallel. - Provide your answer below:
-
Great Lakes Packing has two bond issues outstanding. The first issue has a coupon nate of 8 percent, matures in 6 years, has a total face value of 35 million, and is quoted at 101.2 percent of face...

Study smarter with the SolutionInn App


