Question: The balanced equation for the reaction of gaseous nitrogen dioxide and fluorine is The experimentally determined rate law is A suggested mechanism for this reaction
The balanced equation for the reaction of gaseous nitrogen dioxide and
fluorine is
![]()
The experimentally determined rate law is
![]()
A suggested mechanism for this reaction is
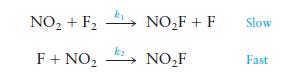
Is this an acceptable mechanism? That is, does it satisfy the two requirements?
2NO(g) + F(g) 2NOF(g)
Step by Step Solution
3.42 Rating (165 Votes )
There are 3 Steps involved in it
The first requirement for an acceptable mechanism is that the sum of ... View full answer

Get step-by-step solutions from verified subject matter experts


