The flask below contains atoms of A (red) and B (yellow). They react as follows 2 A
Question:
The flask below contains atoms of A (red) and B (yellow). They react as follows 2 A (g) + B (g) → A2B(g), with K = 0.25. Draw a picture of the flask and its contents after the reaction has reached equilibrium.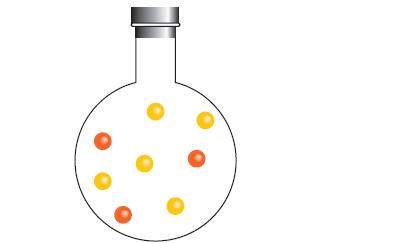
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Vapor pressure increases because of the increased ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Suppose you are designing a chemical plant that is providing phosphorus compounds to other industries and you need to explore the equilibrium properties for the reaction of PCl 5 (g) PCl 3 (g) + Cl...
-
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these...
-
Find the coordinates of the midpoint of the segment with the given endpoints. P(4, -1) and Q(-2, 3)
-
Which of the following K-value expressions, if any, is (are) rigorous? For those expressions that are not rigorous, cite the assumptions involved. (a) Ki = ФiL/ФiV (b) Ki =...
-
Briefly describe how the use of databases could help in detecting kickback fraud schemes.
-
Chemical vapor deposition (CVD) on an inclined susceptor: a case-study problem. An important application of convective mass transfer theory is in CVD processes employed to coat surfaces with thin...
-
1. What is Corvinos criticism of the term unnatural? 2. What does Corvino think about Aquinas evaluation of adultery, rape, and masturbation? 3. What does Corvino think about celibacy of Aquinas and...
-
The field of project management is extremely complex. To be successful, a project manager must be familiar with a variety of tools, methodologies, and information. The definition of a project's...
-
You are required to submit a report that explains with a sufficient level of detail how the results are obtained. Also, you need to submit your codes (if any). Everything should be submitted on BB in...
-
Calculate the solubility in water (in milligrams per liter) of (a) Air at 0.80 atm; (b) He at 0.80 atm; (c) He at 36 kPa. The temperature is 20C in each case, and the pressures are partial pressures...
-
The vapor pressure of benzene is 100.0 Torr at 26 C. A nonvolatile compound was added to 0.400 mol C 6 H 6 (l) at 26C and the vapor pressure of the benzene in the solution decreased to 68.0 Torr....
-
(a) Use the Numerov method to find all the bound-state eigenvalues for a particle in a rectangular well (Section 2.4) of length l with V0 = 20h2/ml2. Note that V is different in different regions and...
-
Carnoustie Capital Management, Ltd. (CCM), a UK-based global investment advisory firm, is considering adding an emerging market currency product to its offerings. CCM has for the past three years...
-
Based on her investigation, Al-Khalili would most likely recommend: A. active currency management. B. a hedging ratio closer to 100%. C. a narrow discretionary band for currency exposures. Kalila...
-
Which of Yellows statements regarding the factors affecting the selection of a trading strategy is correct? A. Statement 1 B. Statement 2 C. Statement 3 Robert Harding is a portfolio manager at...
-
Discuss two advantages of Hedge Fund B relative to Hedge Fund C with respect to investment characteristics. Sushil Wallace is the chief investment officer of a large pension fund. Wallace wants to...
-
Given the parameters for the benchmark given by Harding, Yellow should recommend a benchmark that is based on the: A. arrival price. B. time-weighted average price. C. volume-weighted average price....
-
How would the companys cash needs change for the company in Examples 14-1 to 14-5 if the third project started in May instead of February and the retention was not released until the following year?
-
White Bolder Investments (WBI) You are an intern working for WBI, a large investment advisory services in Sydney. Among other regular customers, WBI has been providing advisory services for Jumbo...
-
Draw resonance structures for each of the following anions. a. b. c.
-
In the reversible adiabatic expansion of 1.75 mol of an ideal gas from an initial temperature of 27.0C, the work done on the surroundings is 1300. J. If C V ,m = 3/2R, calculate q, w, U, and H.
-
For a given set of conditions, the fugacity of a gas is greater than the pressure. What does this tell you about the interaction between the molecules of the gas?
-
5. For each matrix, find the eigenvalues and eigenvectors by hand (no computers please!). Then write down a matrix S such that S-AS (or S-BS) is diagonal, and the resulting diagonal matrix. 20 2 a) A...
-
Find the effective rate of interest of an investment that earns 3.73% compounded quarterly. (1year = 365days). Round the effective rate to two decimal places. NOM= EFF= C/Y =
-
Use synthetic division to divide the two polynomials. 1 W+7 3 ) 4 W 45 5 86 3 W4. 3 - 47 w-35w2-w-21 3 Synthetic Divisi

Study smarter with the SolutionInn App


