Question: The four flasks below were prepared with the same volume and temperature. Flask I contains He atoms, Flask II contains Cl 2 molecules, Flask III
The four flasks below were prepared with the same volume and temperature. Flask I contains He atoms, Flask II contains Cl2 molecules, Flask III contains Ar atoms, and Flask IV contains NH3 molecules. Which flask has
(a) The largest number of atoms;
(b) The highest pressure;
(c) The greatest (mass) density;
(d) Molecules or atoms with the highest root mean square speed;
(e) Molecules or atoms with the highest molar kinetic energy?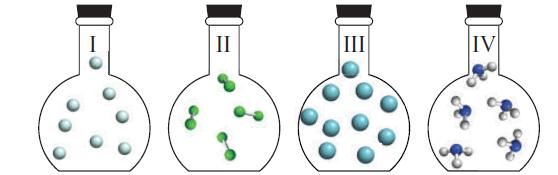
II III IV
Step by Step Solution
3.43 Rating (153 Votes )
There are 3 Steps involved in it
Assuming all the flasks have the same volume and temperature a The largest number of atoms Flask I w... View full answer

Get step-by-step solutions from verified subject matter experts


