The four gases NH 3 , O 2 , NO, and H 2 O are mixed in
Question:
The four gases NH3, O2, NO, and H2O are mixed in a reaction vessel and allowed to reach equilibrium in the reaction 4 NH3(g) + 5 O2(g) ⇌ 4 NO(g) + 6 H2O(g). Certain changes (see the following table) are then made to this mixture. Considering each change separately, state the effect (increase, decrease, or no change) that the change has on the original equilibrium values of the quantity in the second column (or K, if that is specified). The temperature and volume are constant.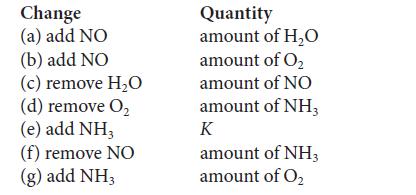
Transcribed Image Text:
Change (a) add NO (b) add NO (c) remove H₂O (d) remove O₂ (e) add NH3 (f) remove NO (g) add NH3 Quantity amount of H₂O amount of O₂ amount of NO amount of NH3 K amount of NH3 amount of O₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a The amount of ...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The four substances HCl, I 2 , HI, and Cl 2 are mixed in a reaction vessel and allowed to reach equilibrium in the reaction 2 HCl(g)+ I 2 (s) 2 HI(g)+ Cl 2 (g). Certain changes (which are specified...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Phosphorus pentachloride, PCl 5 , is used to convert alcohols (such as CH 3 CH 2 OH) to alkyl chlorides (such as CH 3 CH 2 Cl). If you were an industrial chemist, you might be asked to prepare some...
-
Go to the 2013 annual report for Kelloggs Company at http://investor.kelloggs.com/investor-relations/annual-reports. What is the cost of finished goods inventory for 2013 as shown in note 17?
-
Before switch S in Figure is closed, the voltage across the terminals of the switch is 120 V and the voltage across the 0.2 ?F capacitor is 40 V. The total energy stored in the two capacitors is 1440...
-
Do you think Emma was correct in her decision not to carry out interviews? Give reasons for your answer. Emma was now at the start of her final year of her business and accounting degree. This was a...
-
Extend the analysis of heat transfer over a wedge flow. Derive the following equation for the temperature profile: \[\begin{equation*}\theta^{\prime \prime}+(m+1) \operatorname{Prf} \theta^{\prime}=0...
-
Blue Corporation donates the following property to The Corporate Income Tax? Corporations Johnson Elementary School: XYZ Corporation stock purchased two years ago for $25,000. The stock has a...
-
You're planning a trip to France. The current exchange rate is 1.21 dollars per euro. If you want to get 3,000, how many dollars do you have to pay? If you want to exchange $3,000, how many euros...
-
You talk to Sally about getting paid for the work you're doing. You suggest $25 an hour and she agrees. You are only doing this temporarily since you have some extra time so you set yourself up as a...
-
You are now a chemical engineer in the process of designing a plant to separate hydrocarbons obtained from crude oil. You need to keep track of the composition of the mixtures you are dealing with,...
-
Suppose you are a chemical engineer working in a plant that produces hydrogen iodide and are exploring the efficiency of the production process. If you know the value of K, for any given composition...
-
The area between curve y = x(4 x) and the x-axis is rotated through 360 about the x-axis. Find the exact value of the volume of the solid of revolution formed by this rotation.
-
Give two distinct realizations for the transfer functions below: (a) \(H(z)=0.0034+0.0106 z^{-2}+0.0025 z^{-4}+0.0149 z^{-6}\). (b) \(H(z)=\left(\frac{z^{2}-1.349 z+1}{z^{2}-1.919 z+0.923}...
-
Depending on your age and work history, you may have already received this report from the SSA. How well do you understand how your own future retirement benefits will work? Consider other examples...
-
Some FIR filters present a rational transfer function: (a) Show that the transfer function \[H(z)=\frac{\left(r^{-1} z ight)^{-(M+1)}-1}{r e^{\mathrm{j} 2 \pi /(M+1)} z^{-1}-1}\] corresponds to an...
-
Show that: (a) If \(H(z)\) is a Type I filter, then \(H(-z)\) is Type I. (b) If \(H(z)\) is a Type II filter, then \(H(-z)\) is Type IV. (c) If \(H(z)\) is a Type III filter, then \(H(-z)\) is Type...
-
Calculate DVA in Example 24.6. Assume that default can happen in the middle of each month. The default probability of the bank is 0.001 per month for the two years and the recovery rate in the event...
-
Determine the break-even contribution margin ratio for a company with a fixed overhead of $92,000 and revenues of $450,000.
-
Q:1 Take any product or service offered in Pakistan and apply all determinents of customer Perceived value ?
-
Using compounds that possess no more than two carbon atoms, propose a plausible synthesis for the following compound.
-
Assign an IUPAC name for each of the following compounds. a. b. c. d. e. f. g. SH
-
Predict the products that are expected when each of the following compounds is heated with concentrated HBr. a. b. c. d.
-
A 0.5-m 3 rigid tank contains air initially at 1000 kPa and 22C. Air at 2000 kPa and 22C is slowly added until the pressure reaches 1500 kPa. Assume that the temperature in the tank does not change...
-
A piston-cylinder device contains water at a pressure of 400.0 kPa and temperature of 300.0, at an initial volume of 2.500 m^3. The system undergoes a process at constant pressure that brings the...
-
A heavy truck with a weight/horsepower ratio of 300 lb/hp has an initial speed of 55 mph on a 0% grade. The truck then enters a 5% grade which is 8,500 ft. long. How many feet after entering the 5%...

Study smarter with the SolutionInn App


