The height of a column of liquid that can be supported by a given pressure is inversely
Question:
The height of a column of liquid that can be supported by a given pressure is inversely proportional to its density. An aqueous solution of 0.010 g of a protein in 10. mL of water at 20°C shows a rise of 5.22 cm in the apparatus shown in Fig. 5F.3. Assume the density of the solution to be 0.998 g · cm–3 and the density of mercury to be 13.6 g · cm–3.
(a) What is the molar mass of the protein?
(b) What is the freezing point of the solution?
(c) Which colligative property is best for measuring the molar mass of these large molecules? Give reasons for your answer.
FIGURE 5F.3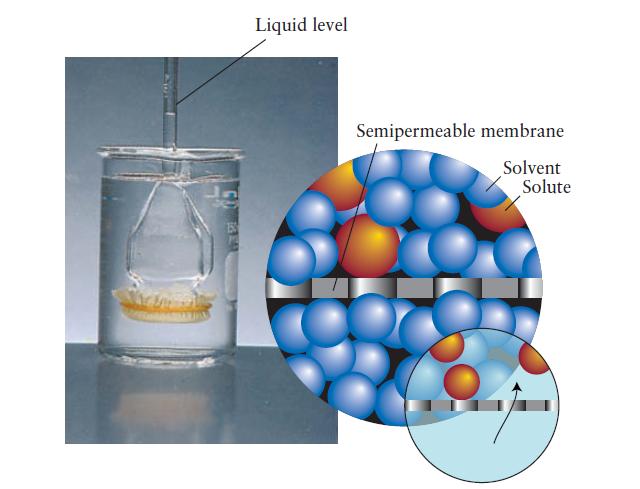
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





