Question: The table below lists the cell potentials for the 10 possible galvanic cells assembled from the metals A, B, C, D, and E and their
The table below lists the cell potentials for the 10 possible galvanic cells assembled from the metals A, B, C, D, and E and their respective 1.00 M 21 ions in solution.
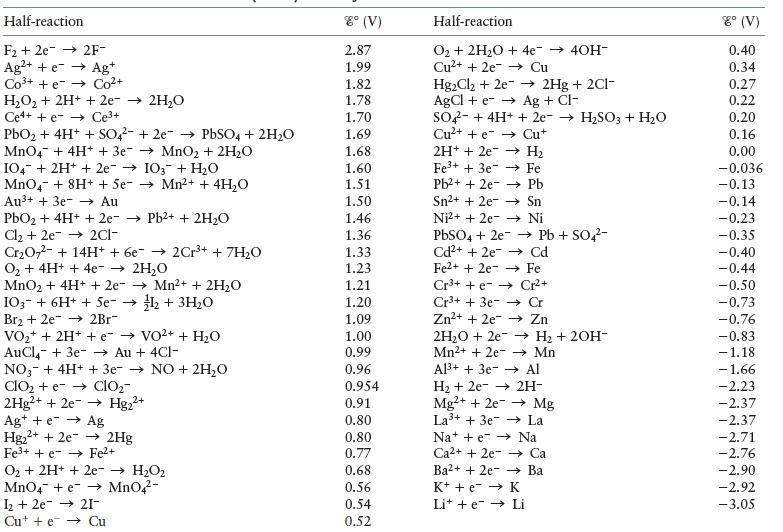
Using the data in the table, establish a standard reduction potential table similar to Table 11.1 in the text. Assign a reduction potential of 0.00 V to the half-reaction that falls in the middle of the series. You should get two different tables. Explain why and discuss what you could do to determine which table is correct.
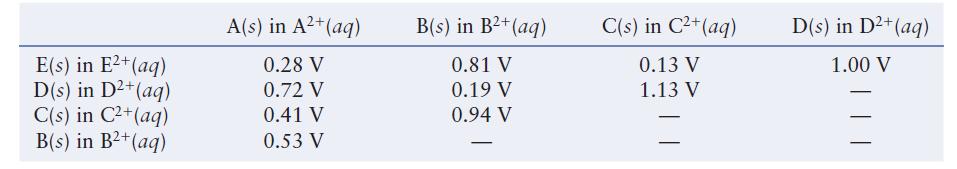
Table 11.1
E(s) in E+ (aq) D(s) in D+ (aq) C(s) in C+ (aq) B(s) in B+ (aq) A(s) in A+ (aq) 0.28 V 0.72 V 0.41 V 0.53 V B(s) in B+ (aq) 0.81 V 0.19 V 0.94 V C(s) in C+ (aq) 0.13 V 1.13 V D(s) in D+ (aq) 1.00 V
Step by Step Solution
3.45 Rating (168 Votes )
There are 3 Steps involved in it
To establish a standard reduction ... View full answer

Get step-by-step solutions from verified subject matter experts


