The titration of (mathrm{Na}_{2} mathrm{CO}_{3}) with (mathrm{HCl}) has the following qualitative profile: a. Identify the major species
Question:
The titration of \(\mathrm{Na}_{2} \mathrm{CO}_{3}\) with \(\mathrm{HCl}\) has the following qualitative profile:
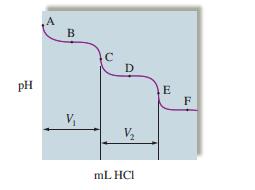
a. Identify the major species in solution as points A-F.
b. For the titration of \(25.00 \mathrm{~mL}\) of \(0.100 \mathrm{M} \mathrm{Na}_{2} \mathrm{CO}_{3}\) with \(0.100 \mathrm{M} \mathrm{HCl}\), calculate the \(\mathrm{pH}\) at points A-E. (B and D are halfway points to equivalence.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





