Question: The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute. If the solute is less
The two beakers in the sealed container illustrated below contain pure water and an aqueous solution of a volatile solute. If the solute is less volatile than water, explain what will happen to the volumes in the two containers as time passes.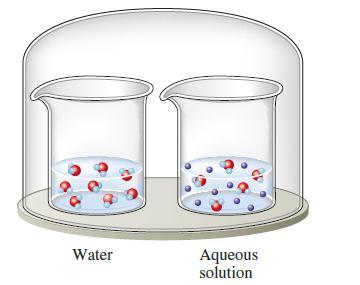
Water Aqueous solution
Step by Step Solution
★★★★★
3.42 Rating (168 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

The volume of the aqueous solution will decrease as the solute evaporates The volume o... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


