Three mechanisms for the reaction NO 2 (g) + CO(g) CO 2 (g) + NO(g) have
Question:
Three mechanisms for the reaction NO2(g) + CO(g) → CO2(g) + NO(g) have been proposed: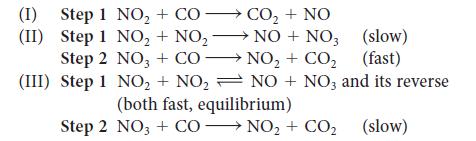
Which mechanism agrees with the following rate law: Rate = kr[NO2]2? Explain your reasoning.
Transcribed Image Text:
Step 1 NO₂ + Step 1 Step 2 (III) Step 1 (1) (II) Step 2 COCO₂ + NO NO + NO3 NO₂ CO₂ NO+ NO3 and its reverse NO₂ + NO₂ NO3 + CO- NO₂ + NO₂ (both fast, equilibrium) (slow) (fast) NO3 + CONO₂+ CO₂ NO₂ + CO₂ (slow)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
If mechanism I were correct the rate law would be rate k ...View the full answer

Answered By

Diane Joyce Pastorin
Please accept my enthusiastic application to solutioninn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In addition to vision system using visual light, scientist has invented new systems with capabilities beyond human visions. Discuss five latest technologies that able to map the unseen world and...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The reaction 2NO(g) + O2(g) 2NO2(g) exhibits the rate law Rate = k[NO]2[O2] Which of the following mechanisms is consistent with this rate law? a. NO + O2 NO2 + O Slow O + NO NO2 Fast b. NO + O2 ...
-
What is the maximum height above ground that a projectile of mass 0.790 kg, launched from ground level, can achieve if you are able to give it an initial speed of 80.3 m/s?
-
Explain how it might be possible to build an OS that uses full segmentation, but has no file system.
-
Diderot Drilling Company has leased property on which oil has been discovered. Wells on this property produced 18,000 barrels of oil during the past year that sold at an average sales price of $55...
-
Show that the variance of a factorial effect in a $2^{k}$ design with $m$ replications is \[\frac{4 \sigma^{2}}{N}\] where $N=m \cdot 2^{k}$ and $\sigma^{2}$ is the variance of each outcome.
-
A computer IC chip consumes 10 W of power, which is dissipated as heat. The chip measures 4 cm by 4 cm on a side and is 0.5-cm thick. Currently, the IC chip is packaged into an electronic device as...
-
Part 1: Email Format Give an email to your instructor to ask for a meeting. Use your instructor's full name in the TO: line Use the format, structure and design Course Competencies from the course to...
-
Dinitrogen pentoxide, N 2 O 5 , decomposes by a first-order reaction. What is the initial rate of decomposition of N 2 O 5 when 3.45 g of N 2 O 5 is confined in a container of volume 0.750 L and...
-
For the first-order reaction A 3 B + C, when [A] 0 = 0.015 mol L 1 , the concentration of B increases to 0.018 mol L 1 in 3.0 min. (a) What is the rate constant for the reaction expressed as the...
-
The California Healthy Kids Survey in 1999 and 2001 asked 7th, 9th, and 11th graders in San Luis Obispo County whether they smoked tobacco. In 1999, 6% of 7th graders, 17% of 9th graders, and 23% of...
-
At Enron Energy Services, many of the contracts were overvalued, as the energy loads required by its customers were based on guesswork and excessively optimistic assumptions. True/False
-
WorldCom improperly accounted for its line cost expense by: (a) Allocating line-cost expense to PPE in its first recording of the line-cost transactions. (b) Initially properly debiting the line-cost...
-
If a parent company discovers that the assets of an acquired company are worth less than it believed at the time of the acquisition, it is appropriate to increase goodwill because this means that the...
-
Complete the MPS record shown in Figure 11.30 for a single item. Item: A Quantity on Hand: Forecast Customer orders (booked) Projected on-hand inventory MPS quantity MPS start 75 1 65 January 2 65 40...
-
The decline of a companys asset-turnover ratio is an indication that assets are impaired. True/False
-
Jim Macklin is chief executive officer of Red Cliff Machinery, Inc. The company adopted a JIT operating environment five years ago. Since then, each segment of the company has been converted, and a...
-
The Thomas Corporation was organized on Jan. 1, 2020. On Dec. 31, 2021, the corporation lost most of its inventory in a warehouse fire before the year-end count of inventory was to take place. just...
-
Identify which arrow-pushing pattern is utilized in the following step:
-
For each of the following multistep reactions, read the curved arrows and identify the sequence of arrowpushing patterns: a. b. c. d. e. :OH 0=s=0. :H 0=s=0: o=s=0:
-
The following two reactions will be explored in different chapters. Yet, they are very similar. Identify and compare the sequences of arrow-pushing patterns for the two reactions. Reaction 1 Reaction...
-
What does a row correspond to in this data table how would you best describe its role?
-
Explain the causes behind regional inequality in India and the probable connective measures.
-
When dealing with other workers, provide an example of how to respect their differences in culture.

Study smarter with the SolutionInn App


