To generate the starting material for a polymer that is used to make water bottles, hydrogen is
Question:
To generate the starting material for a polymer that is used to make water bottles, hydrogen is removed from the ethane in natural gas to produce ethene in the catalyzed reaction C2H6(g) → H2(g) + C2H4(g). Use the information in Appendix 2A to calculate the equilibrium constant for the reaction at 298 K.
(a) If the reaction is begun by adding the catalyst to a flask containing C2H6 at 40.0 bar, what will be the partial pressure of the C2H4 at equilibrium?
(b) Identify three steps the manufacturer can take to increase the yield of product.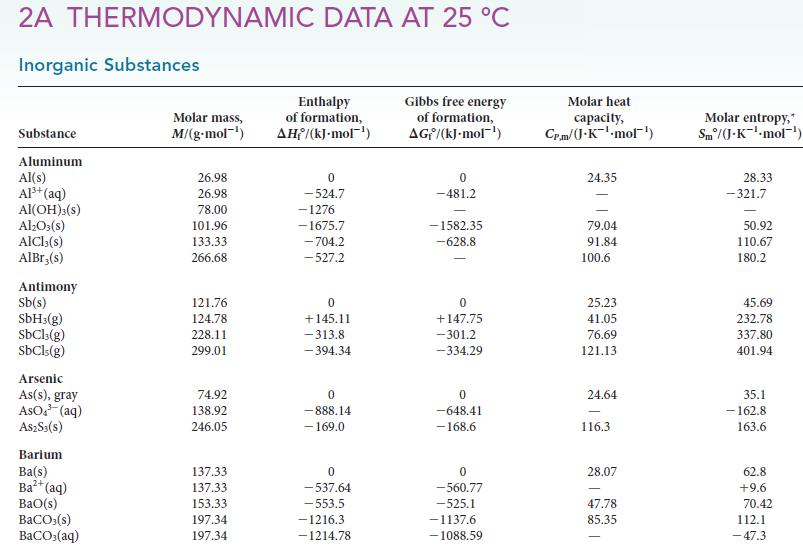
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





