Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the reaction enthalpies for the halogenation of ethene
Question:
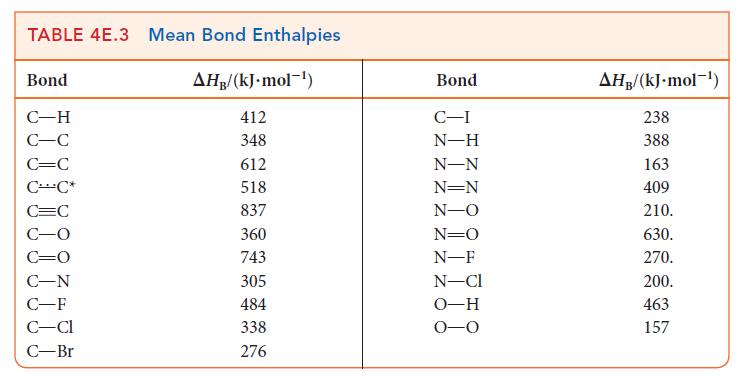
Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the reaction enthalpies for the halogenation of ethene by chlorine, bromine, and iodine. What trend, if any, exists in these values?
Transcribed Image Text:
TABLE 4E.2 Bond Enthalpies of Diatomic Molecules Molecule H N 0 CO F Cl Br 1 HF HCI HBr HI AHB/(kJ.mol-) 436 944 496 1074 158 242 193 151 565 431 366 299
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 20% (5 reviews)
CH4 XCH4X break one XX bond and form two CX bonds Using bond enthalpies Haloge...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use bond enthalpies (Tables 4E.2 and 4E.3) to estimate the reaction enthalpies for the hydrohalogenation of ethene by HX, where X = Cl, Br, I. What trend, if any, exists in these values? TABLE 4E.2...
-
Use the data in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) N(g) + 3 F(g) 2 NF3(g) (b) CH3CHCH(g) + HO(g) CH3CH(OH)CH3(g) (c) CH4 (g) + Cl(g) CHCl(g) + HCl(g)
-
Use the bond enthalpies in Tables 4E.2 and 4E.3 to estimate the reaction enthalpy for (a) 3 CH(g) CH(g) (b) CH4(g) + 4 Cl(g) CCl4(g) + 4 HCl(g) (c) CH4(g) + CCl4(g) CHCl3(g) + CH3CI(g)
-
Amandeep, a graduate of Cambrian Colleges Business Program, found a job as an online marketing specialist for Tangerine Bank. Within a year, he had saved {A} and decided to buy a new car but was not...
-
Find vo(t) and io(t) in the network infigure. i,(1) 0.01 F 0.02 F: 25 cos20r v (+ 0.4 H 5ag v,1) 503
-
Ashton Fleming has asked you to document the cash receipts system at S&S. Ashtons narrative of the system follows: Customer payments include cash received at the time of purchase and payments...
-
The race track is a fascinating example of financial market dynamics at work. Let's go to the track and make a wager. Suppose that, from a field of 10 horses, we simply want to pick a winner. In the...
-
Presented below is a list of balance sheet accounts in alphabetical order. Accounts payable .................. Inventories Accounts receivable ................. Land (in use) Accumulated...
-
The estimated taxable income for the year ended 31 December 2021 is R8 425 000. What is the 1st provisional payment amount?
-
Write the formula of (a) Methyl propyl ether; (b) Ethyl butyl ether; (c) Dipropyl ether.
-
Identify the type of copolymer formed by monomers A and B: BBBBAA.
-
For the following data: (a) Find the mean, variance, and standard deviation. (b) Construct a frequency table with five classes. (c) Using the grouped data formula, find the mean, variance, and...
-
Ten examples of products and services that are likely to appeal to typical households in M3A 3B3 postal area, based on the demographic/lifestyle characteristics. (Please choose a variety of different...
-
Analyze the Monitoring and Controlling processes identified in the scrum methodology. Identify and analyze the project evaluation system in your selected scrum methodology (If there is no evaluation...
-
Several sectors in the global economy have been hit especially hard due to the COVID-19 pandemic. A couple of the hardest-hit sectors include automotive, healthcare services, pharma, hospitality, and...
-
1- Name an industry or a type of business that would be most open to the use of self-managing teams. Then, name an industry or a type of business that would be least open to the use of self-managing...
-
Using your own company accomplish the following: I. Company Name and Its Background (give brief description: the company background, its firmographic profile, business operations, summary only) II....
-
Taxes in Oz are calculated according to the formula T = .01/2 Where T represents thousands of dollars of tax liability and I represents income measured in thousands of dollars. Using this formula,...
-
In a certain school district, 3% of the faculty use none of their sick days in a school year. Find the probability that 5 faculty members selected at random used no sick days in a given year.
-
Identify the repeating unit of the polymer formed from each of the following reactions, and then determine whether the polymer is a chain-growth or a step-growth polymer. (a) (b) (c) NH2 - [] - NH,...
-
Nitroethylene undergoes anionic polymerization so rapidly that it is difficult to isolate nitroethylene without it polymerizing. Explain.
-
Identify which of the following monomers would be most reactive toward anionic polymerization: CH3 OAc CN
-
What is Recruitment? Explain the factors affecting Recruitment.
-
Find the values of a, b, c, and d which satisfy the matrix equation. [a+c a+2b [0 -7] e-1 4d-6 13 2d
-
Explain the function of the Ping Utility. John has a PC with the IP Address 192.168.21.10/24. He tries to ping Jane's PC which has 192.168.20.11/24 as its IP Address and gets no reply. Explain the...

Study smarter with the SolutionInn App


