Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for (a) The freezing
Question:
Use data in Table 4C.1 or Appendix 2A to calculate the entropy change for
(a) The freezing of 1.00 mol H2O(l) at 0.00°C;
(b) The vaporization of 50.0 g of ethanol, C2H5OH, at 351.5 K.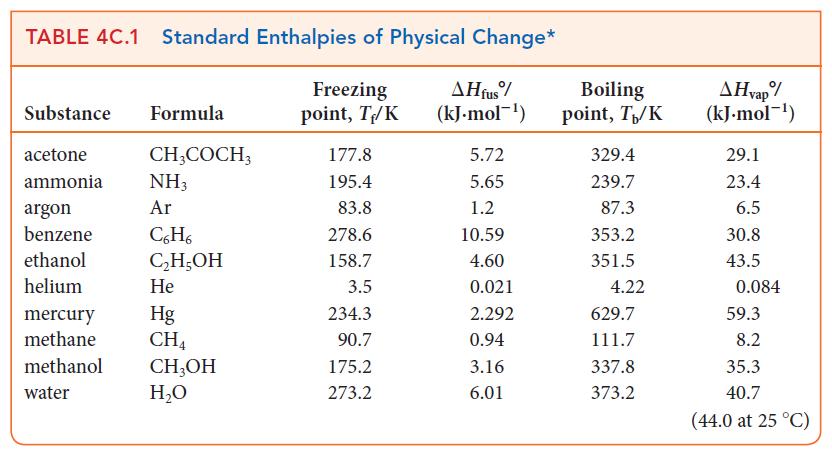
Transcribed Image Text:
TABLE 4C.1 Standard Enthalpies of Physical Change* Freezing AH fus% (kJ.mol-¹) point, T/K Substance Formula acetone ammonia argon benzene ethanol helium CH3COCH3 NH3 Ar C6H6 C₂H5OH He mercury Hg methane CH4 methanol CH3OH water H₂O 177.8 195.4 83.8 278.6 158.7 3.5 234.3 90.7 175.2 273.2 5.72 5.65 1.2 10.59 4.60 0.021 2.292 0.94 3.16 6.01 Boiling point, T₁/K 329.4 239.7 87.3 353.2 351.5 4.22 629.7 111.7 337.8 373.2 AHvap% (kJ.mol-¹) 29.1 23.4 6.5 30.8 43.5 0.084 59.3 8.2 35.3 40.7 (44.0 at 25 °C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a 2...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard reaction entropy for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction...
-
Use data in Table 4H.1 or Appendix 2A to calculate the standard entropy change for each of the following reactions at 25C. For each reaction, interpret the sign and magnitude of the reaction entropy....
-
Calculate the entropy change for the vaporization of liquid methane and hexane using the following data: Compare the molar volume of gaseous methane at 112 K with that of gaseous hexane at 342 K. How...
-
How do items discussed in the critical audit matters section differ from items in an unqualified opinion with an emphasis-of-matter paragraph? Question 1: (20) A. On November 1, 2019. James Andersun...
-
(a) What source documents are used in assigning (1) materials and (2) labor to production in a process cost system? (b) What criterion and basis are commonly used in allocating overhead to processes?
-
Find the angle between the front legs and the back legs of the folding chair shown in Fig. 9.75. Fig. 9.75 21 in. 25 in. 19 in.
-
For each of the following processes, determine what energy conversion takes place and classify the interaction as dissipative or nondissipative. (a) The launching of a ball by the expanding of a...
-
List two examples of audit evidence the auditor can use in support of each of the following: a. Recorded amount of entries in the acquisitions journal b. Physical existence of inventory c. Accuracy...
-
G is an individual GST registrant that files GST returns annually. At the beginning of year 20XX, G incorporated their sole proprietorship into G Inc., a wholly owned subsidiary of G, and registered...
-
Calculate the work for each of the following processes beginning with a gas sample in a piston assembly with T = 305 K, P = 1.79 atm, and V = 4.29 L: (a) Irreversible expansion against a constant...
-
What might you expect the residual molar entropy of trigonal pyramidal PH 2 F to be if it were to adopt an orientationally disordered arrangement in its crystal form?
-
Use the information for Prosperous Bank in Exercise 25 to compute the total cost for each revenue-generating area using the algebraic method.
-
Bree is an officer of Chic Petites Corporation. Like most corporate officers, Bree a. can act as Chics agent. b. can participate in managing Chics day-to-day operations. c. must carry out the duties...
-
What is involved in an agents duty of loyalty?
-
What are some of the partnership rights of partners?
-
A writing is always necessary to form a partnership. (True/False)
-
Under what circumstances is a principal liable to a third party on a contract entered into by an agent?
-
In order to get a license to practice in the United States, foreign-trained veterinarians must take an exam given by the American Veterinary Association. Only 48 people per year are allowed to take...
-
In the simple quantity theory of money, what will lead to an increase in aggregate demand? In monetarism, what will lead to an increase in aggregate demand?
-
In 1994 chemists at Texas A&M University reported the synthesis of a non-naturally occurring amino acid: a. To which naturally occurring amino acid is this compound most similar? b. A tetrapeptide,...
-
Consider the following reactions at some temperature: 2NOCl( g) 2NO( g) + Cl2( g) K = 1.6 1025 2NO( g) N2( g) + O2( g) K = 1 1031 For each reaction some quantities of the reactants were placed in...
-
Consider the following reaction: H2O( g) + CO(g) H2( g) + CO2(g) Amounts of H2O, CO, H2, and CO2 are put into a flask so that the composition corresponds to an equilibrium position. If the CO placed...
-
A company estimates that it will need $144,000 in 16 years to replace a computer. If it establishes a sinking fund by making fixed monthly payments into an account paying 6.3% compounded monthly, how...
-
Write the expression as one logarithm. 9 loga x + 43 loga(x-9) 10 loga (5x + 2)
-
K Solve the equation analytically. 22x-1= 16 The solution set is (Simplify your answer.)

Study smarter with the SolutionInn App


