Question: Use Fig. 5B.2 to predict the phase of a sample of water under the following conditions: (a) 1 atm, 200C; (b) 100. atm, 50.0C; (c)
Use Fig. 5B.2 to predict the phase of a sample of water under the following conditions:
(a) 1 atm, 200°C;
(b) 100. atm, 50.0°C;
(c) 3 Torr, 10.0°C.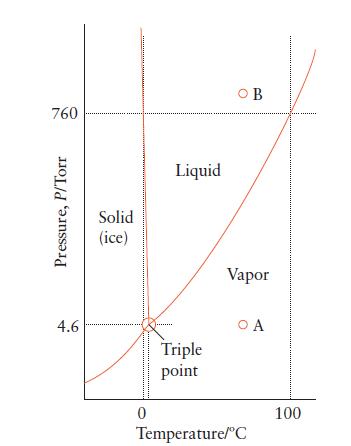
760 Pressure, P/Torr 4.6 Solid (ice) 0 Liquid Triple point OB Vapor O A Temperature/C 100
Step by Step Solution
3.38 Rating (148 Votes )
There are 3 Steps involved in it
a Va... View full answer

Get step-by-step solutions from verified subject matter experts


