Use the phase diagram for carbon in Exercise 5B.4 (a) To describe the phase transitions that carbon
Question:
Use the phase diagram for carbon in Exercise 5B.4
(a) To describe the phase transitions that carbon would undergo if the pressure on a sample is increased at a constant temperature of 2000 K from 100 atm to 1 * 106 atm;
(b) To rank the diamond, graphite, and liquid phases of carbon in order of increasing density.
Exercise 5B.4
The phase diagram for carbon, shown here, indicates the extreme conditions that are needed to form diamonds from graphite.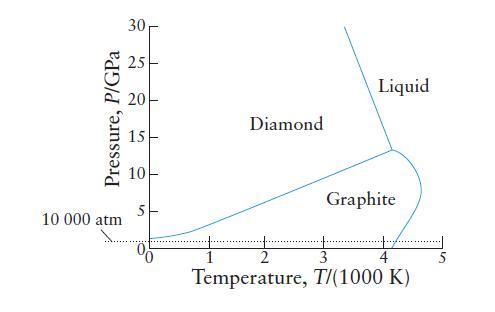
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





