Question: Mutual solubility data for the isooctane (1)/furfural (2) system at 25?C are [Chem. Eng. Sci., 6, 116 (1957)] Compute: (a) The distribution coefficients for isooctane
Mutual solubility data for the isooctane (1)/furfural (2) system at 25?C are [Chem. Eng. Sci., 6, 116 (1957)]
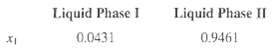
Compute:
(a) The distribution coefficients for isooctane and furfural
(b) The relative selectivity for isooctane relative to furfural
(c) The activity coefficient of isooctane in liquid phase 1 and the activity coefficient of furfural in liquid phase 2 assuming ?2(1)) = 1.0 and ?1(1) = 1.0.
Liquid Phase 1 0.0431 Liquid Phase II 0.9461
Step by Step Solution
3.46 Rating (162 Votes )
There are 3 Steps involved in it
From summation of mole fractions in each liquid phase x 2 in phase I ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (32).docx
120 KBs Word File


