Question: Using the K a K a values given in Table 7.2, calculate the concentrations of all species present and the p H p H for
Using the Ka values given in Table 7.2, calculate the concentrations of all species present and the pH for each of the following.
a. 0.20MHOCl
b. 1.5MHOC6H5
c. 0.020MHF
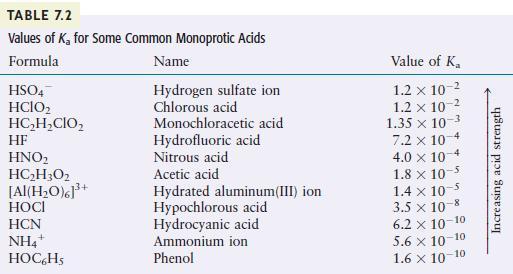
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10-5 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
The question involves calculating the concentrations of all species present and the pH for different solutions of monoprotic acids using given Ka valu... View full answer

Get step-by-step solutions from verified subject matter experts


