Question: Data: Complete the following exercises. 1) Write the general rate law for the equation: Acetone(C3H6O(aq))+Iodine(I2(aq))HCl(aq)C3H5OI(aq)+I(aq)Ratc=K(a(ctone][H+][[2]2 2) Rewrite the general rate law using the concentrations and

![for the equation: Acetone(C3H6O(aq))+Iodine(I2(aq))HCl(aq)C3H5OI(aq)+I(aq)Ratc=K(a(ctone][H+][[2]2 2) Rewrite the general rate law using the](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f85a18f0e11_02466f85a18844df.jpg)
Data:
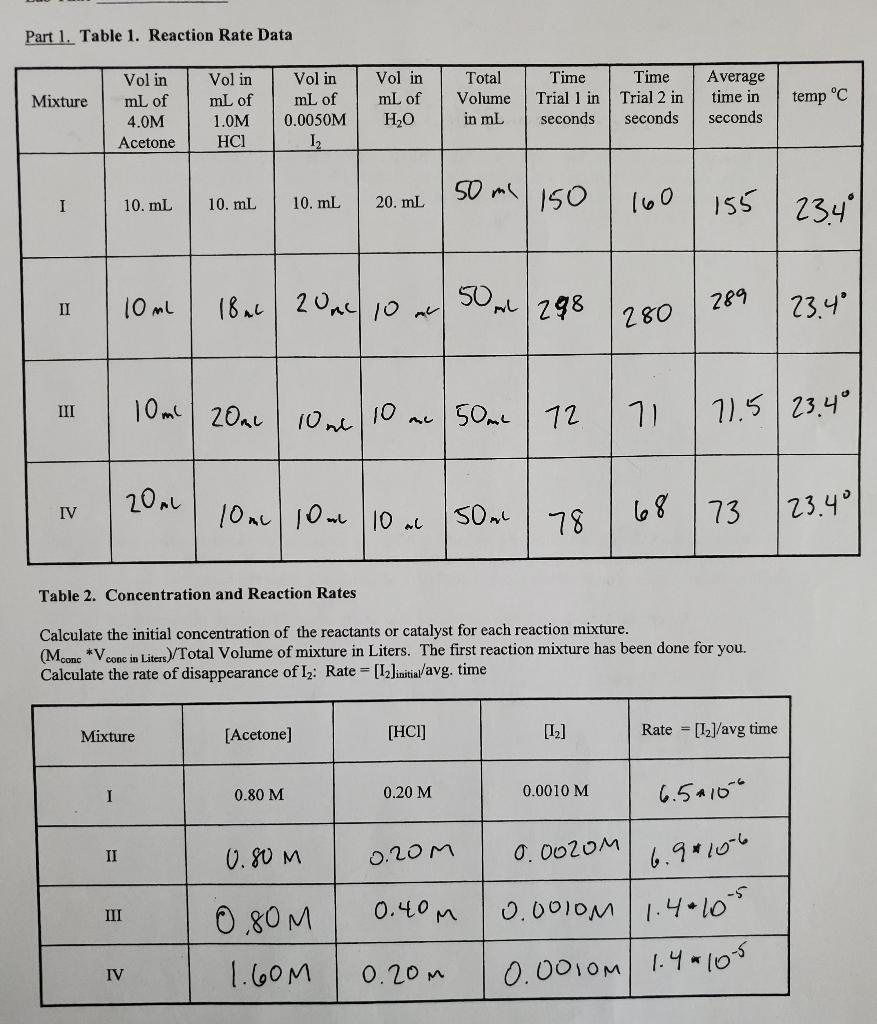
Complete the following exercises. 1) Write the general rate law for the equation: Acetone(C3H6O(aq))+Iodine(I2(aq))HCl(aq)C3H5OI(aq)+I(aq)Ratc=K(a(ctone][H+][[2]2 2) Rewrite the general rate law using the concentrations and the rate values found in Table 2. Your unknowns are the exponents x,y, and z and the rate constant k. You will have 4 equations. For example, the rate law for the equation given in the introduction would be Rate 1=2.4103M/s=k[0.20]x[0.10]y) Rate 1= Rate 2= Rate 3= Rate 4= 3) Determine the values of the exponents x,y, and z. Write the complete ratio comparing one reaction mixture to another. Remember to compare the rates for two mixtures for which only one of the concentrations varies. You must show the cancellation of terms and show the math. a) Determine the exponent x by comparing the rate equations for reaction mixtures 2 and 1 . Rate1=Rate2=M/sM/s=k[0.80]x[0.20]y[0.0010]2k[01.60]x[0.20]y[0.0010]zx=(acetone) b) Determine the exponent y. c) Determine the exponent z. 4) Write the complete rate law by substituting the values of x,y, and z into the general rate law. 5) What is the overall order of the reaction? 6) Complete the rate equation by substituting the exponents x,y and z into the four rate equations. Determine the value of k for each reaction mixture. Show your work and correct units for k. Rate 1k= Rate 3k= Rate 2k= Rate 4k= Avg. k= Part 2. Effect of temperature. Table 3. Using the exponents x,y, and z determined in Part 1, determine the value of k at the temperature of the water bath. What effect did the increase in temperature have on the value of k ? Using the Arrehius equation, determine the Ea in kJ/mol for the iodination of acetone. lnk1k2=REa(T11T21)R=8.314J/(molK) Part 1. Table 1. Reaction Rate Data Table 2. Concentration and Reaction Rates Calculate the initial concentration of the reactants or catalyst for each reaction mixture. (MconcVconcinLiters)/ Total Volume of mixture in Liters. The first reaction mixture has been done for you. Calculate the rate of disappearance of I2 : Rate =[I2]initial/ avg. time
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


