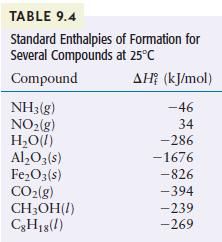
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the
Question:
Using the standard enthalpies of formation listed in Table 9.4, calculate the standard enthalpy change for the overall reaction that occurs when ammonia is burned in air to form nitrogen dioxide and water. This is the first step in the manufacture of nitric acid.
![]()
Transcribed Image Text:
4NH3(g) + 702(g) 4NO(g) + 6HO(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Figure 912 We will use the pathway in which the reactants are broken down into e...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Calculate the heats of combustion for the following reactions from the standard enthalpies of formation listed in Appendix 3: (a) 2H2(g) + O2(g) - 2H2O(l) (b) 2C2H2(g) + 5O2(g) - 4CO2(g) + 2H2O(l)
-
Calculate the heats of combustion for the following reactions from the standard enthalpies of formation listed in Appendix 3: (a) C2H4(g) + 3O2(g) - 2CO2(g) 1 2H2O(l) (b) 2H2S(g) + 3O2(g) - 2H2O(l) +...
-
Using Figure 7. 4, show how the level of oil imports and the price level would be affected if the country represented in that figure acted to internalize national security issues, but ignored climate...
-
A really interesting situation occurs for the logistic equation, Equation 4.46 when a = 3.82831 and x1 = 0.51. Show that a three cycle occurs with the approximate x values 0.16, 0.52, and 0.96 for...
-
Assume that tables T1 and T2 have a 1:1 relationship. Assume that T2 has the foreign key. Show the SQL statements necessary to move the foreign key to T1. Make up your own names for primary and...
-
Bernardino Santos-Rodiguez was piloting a boat owned by his friend, Raul Viera-Torres, in waters near Guayama, Puerto Rico. The boat was equipped with a hydraulic steering system manufactured by...
-
Selected accounts and related amounts for Gloucester Co. for the fiscal year ended August 31, 2014, are presented in Problem 5-5A. In Problem 5-5A, the following selected accounts and their current...
-
Suppose your starting salary is $50,000. You would like to open a savings account to save for your retirement and any large future expenses. Most Economists agree that you should allocate 20% of your...
-
Methanol (CH 3 OH) is sometimes used as a fuel in high-performance engines. Using the data in Table 9.4, compare the standard enthalpy of combustion per gram of methanol with that of gasoline....
-
Diborane (B 2 H 6 ) is a highly reactive boron hydride that was once considered as a possible rocket fuel for the U.S. space program. Calculate H for the synthesis of diborane from its elements,...
-
What is Says law? How does it relate to the view held by classical economists that the economy generally will operate at a position on its production possibilities curve (Chapter)? Use production...
-
This is not deductible from gross income Group of answer choices Travel expenses on a business trip Transportation expenses from home to the office and from the office to the home Transportation...
-
Cost-effectiveness analysis Group of answer choices reports the incremental cost of improving an outcome. reports the value of improved outcomes divided by the cost of those outcomes. focuses only on...
-
If you are familiar with construction, share with the class what you regularly see as the biggest safety challenge. Examples are great, if you have some to share! If you are not familiar with...
-
The most recent cabinet department that was established occurred in 2002 was the Department of _________________________. The most recent cabinet department that was established occurred in 2002 was...
-
While preparing for the year end on July 31, 2020, the following errors, reflected in the data above, occurred: 1. When counting the year-end inventory, staff members counted items costing $5,000...
-
It is your first day at a new job and you talk about the themes of cost system design. One of your new colleagues asks, If different cost information is used for different purposes, does that mean we...
-
Suppose the concentration of glucose inside a cell is 0.1 mm and the cell is suspended in a glucose solution of 0.01 mm. a. What would be the free energy change involved in transporting 10-o mole of...
-
(a) Use graphing software to plot standard potential against atomic number for the elements of Groups 1 and 2. (b) What generalizations can be deduced from the graph? 2B STANDARD POTENTIALS AT 25 C...
-
Is there any chemical support for the view that hydrogen should be classified as a member of Group 17? Give evidence that supports this view.
-
Write the chemical equation for the reaction between strontium metal and hydrogen gas.
-
Your friend Chloe, who is pregnant, mentioned knowing the three stages of preantal development but lacks clarity about the specific occurrences within each stage. Could you elaborate on the names of...
-
What can you tell from the labor productivity pattern exhibited by the data? What might be the reasons for the pattern?
-
What unique source of value did Netflix have that competitors such as Blockbuster did not?

Study smarter with the SolutionInn App


