Question: Which of the changes listed below would affect the value of the rate constant k? a. Increasing the partial pressure of oxygen gas. b. Changing
Which of the changes listed below would affect the value of the rate constant k?
a. Increasing the partial pressure of oxygen gas.
b. Changing the temperature.
c. Using an appropriate catalyst.
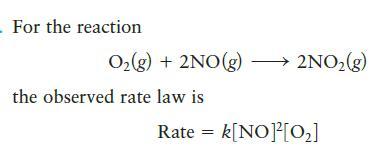
- For the reaction O(g) + 2NO(g) 2NO(g) the observed rate law is Rate = k[NO][0]
Step by Step Solution
3.47 Rating (173 Votes )
There are 3 Steps involved in it
Will the rate constant change if a Increasing the partial pressure of ... View full answer

Get step-by-step solutions from verified subject matter experts


