Question: I need help with question 6, I attached the experiment information. I couldn't find anything that stated why CuSO4 is being used in this experiment.





I need help with question 6, I attached the experiment information. I couldn't find anything that stated why CuSO4 is being used in this experiment. Please help!
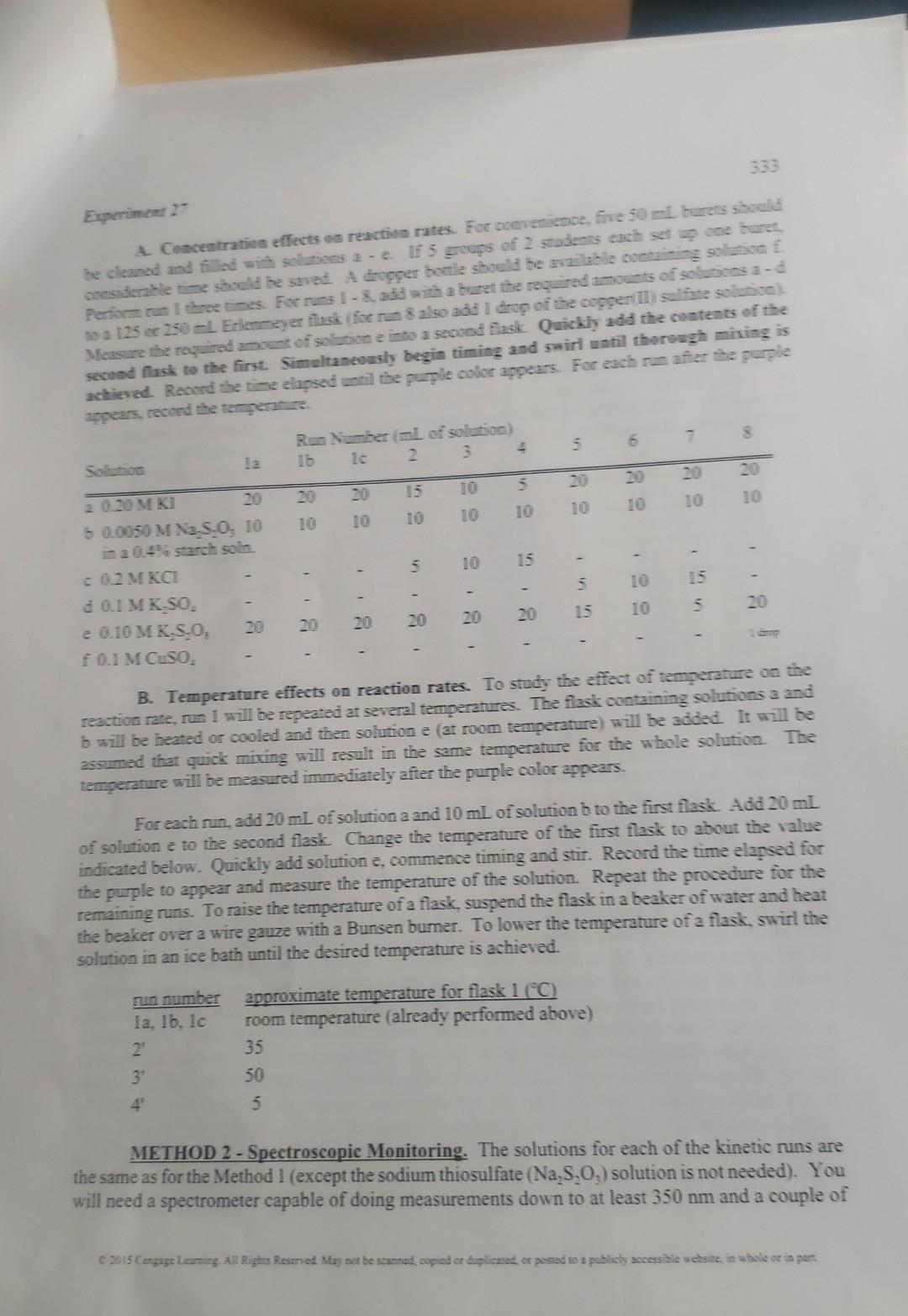
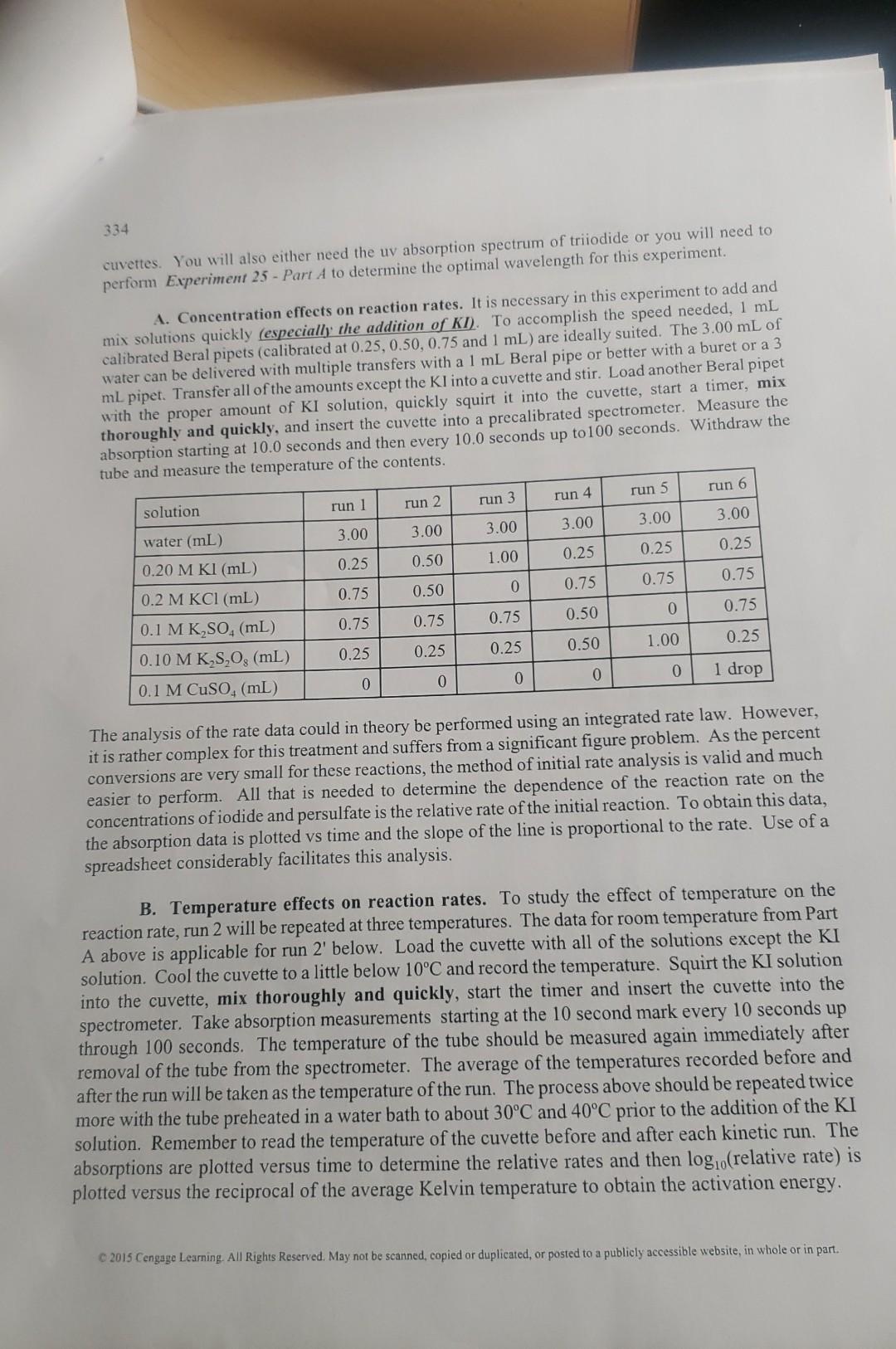
What is the purpose of using the CuSO4 solution in this experiment? Experiment 27 RATES AND MECHANISMS OF REACTIONS - VISUAL AND/OR SPECTROSCOPIC MONITORING Learning Objectives Upon completion of this experiment, students will have experienced: 1. A study of concentration, temperature and a catalyst on reaction rates. 2. An investigation of a reaction mechanism. 3. The derivation of rate law expressions. Text Topics Kinetics, reaction rate expressions, reaction mechanisms, energy of activation, spectroscopy (for correlation with some textbooks, see page ix). Notes to Students and Instructor This experiment demonstrates the general principles of kinetics including the effects of concentrations and temperature on rates of reaction. Two different methods for following the reaction are presented. The first uses a built in clock that leads to a stunning visual indication that the reaction has progressed to a certain percentage of product formation. The second method follows the same reaction but uses spectroscopy in the near ultraviolet to follow the progress of the reaction. The second method is clearly closer to research quality but both approaches compromise on some techniques to allow for the limited time available for the experiment. Discussion Try to visualize a chemical reaction between hydrogen and chlorine to produce hydrogen chloride. When we write the equation on paper, H2(g)+Cl2(g)=2HCl(g), it appears there has been a sudden change from reactants to products. But upon further consideration, we realize that this reaction required several changes. The hydrogen-hydrogen bond and the chlorine-chlorine bond had to break and the hydrogen-chlorine bond had to form. Did these changes occur simultaneously or sequentially? If the latter is the case, in what sequence did they occur? Ideally we would like to be able to videotape the reaction to study the mechanism. Technology that approaches this capability might become available in the future. Currently, for most reactions, the best we can do Q 2015 Cengage Leaming. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 330 is to observe and study the behavior of the reactants and products. We are able to obtain important information about the mechanism of the reaction by studying the effects of concentration and temperature on the rate of the reaction. Reaction rates are also valuable for practical purposes. When adjusting conditions for running an industrial reaction, the chemical engineer wants the reaction to proceed at reasonable rate. If it goes too fast, it might get out of control and overheat or even explode. On the other hand, if it is too slow, it may not be profitable to run the reaction. A knowledge of the dependence of the reaction rate on concentration and temperature helps the chemist to understand the mechanism and the engineer to optimize reaction conditions. The rate of virtually every reaction increases as the temperature increases (as a rough rule of thumb, a 10C increase doubles the reaction rate). Consider the potentially explosive reaction between hydrogen and oxygen to yield water. Although the reaction is highly exothermic, a mixture of hydrogen and oxygen will sit in a bottle indefinitely without noticeable formation of water. Before the oxygens can begin to form bonds with hydrogens, the hydrogen-hydrogen and oxygenoxygen bonds must begin to break. The cleavage of bonds requires energy. This process requires more energy than most of the molecules have at ambient temperature. As the temperature of the system is increased, the fraction of molecules with sufficient energy to undergo bond cleavage upon a collision increases dramatically and the rate of reaction increases. Compare the situation to a bowl containing several marbles. If the bowl is lifted off the ground, the marbles will have potential energy because of their position relative to the ground. But because of the lip of the bowl, the marbles are hindered from returning to the lower energy state. If energy is transferred to the system by shaking the bowl, eventually, if the shaking is hard enough, some of the marbles will attain enough energy to make it over the lip and fall to the ground. In this case, the energy of the marble is transferred to the ground. In the case of the molecules, the energy that is released when product bond formation occurs can often be transferred to unreacted molecules to help them break bonds. The energy barrier between the reactants and products that must be surmounted for the reactants to pass over to products is called the energy of activation. The activation energy can be determined from a study of the reaction rate constant as a function of temperature. lnk=Ea/RT+lnA(k= rate constant at absolute temperature, Ea= energy of activation, R= gas constant, A= constant) The mathematical dependence of the rate of reaction on concentration is more intuitively understandable. Consider again the H2+Cl2 reaction. For the reaction to proceed, a collision between H2 and Cl2 apparently occurs. One would expect that a doubling of either the H2 concentration or the Cl2 concentration would double the number of collisions and the rate. A doubling of both H2 and Cl2 will quadruple the number of collisions and the rate. As the rate is expressed as a change in the amount of a reactant, [H2] per unit time, t, or rate =[H2]t, the rate should show a proportionality to the concentrations of H2 and Cl2 or [H2]/t=k[H2][Cl2] C. 2015 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. Experiment 27 Where k is the proportionality constant that is called the rate constant. The above rate expression is consistent with the conclusions above that a doubling of the concentration of either hydrogen or chlorine wall double the rate. The rate constant, k, actually is temperature dependent (see previous paragraph) and increases markedly with temperature due to an increase in the number and more importantly, the effectiveness of the collisions. The rate expression then for any step of a reaction A2AA+BPPP[A]t=k[A][A]t=2k[A]2[A]t=k[A][B] If the reaction is a multistep reaction, the determination of the rate expression involves a somewhat more complex analysis that is beyond the scope of this discussion. In fact, the system you will study today is a multistep process but the possible rate expressions will be provided to you. Some additives to a reaction mixture markedly increase the reaction rate by providing alternate or lower energy pathways to products. Compounds that increase reaction rates without undergoing any net chemical change themselves are called catalysts. Procedure The stoichiometry of the reaction you will explore today is : 2I+S2O82=I2(aq)+2SO42 This reaction apparently requires the simultaneous collision of three ions. The probability of such an occurrence is very small. Consider the probability of Dylan finding Hope and Carson at the same time. The chance of such a coincidence is small. However, Dylan could encounter one of them and then the two of them could go looking for the third person. Reactions that involve more than two molecules or ions also usually proceed in steps. For the reaction between two iodides and a persulfate, a possible sequence would be the following: I+S2O8=SO42+SO4ISO4S+I=I2(aq)+SO42 mechanism 1 One of these reactions would probably be slower than the other. The slower reaction in a multistep reaction is called the rate determining step. This means that the overall rate for the process is determined primarily by the rate of the slow step. By assuming which step is the rate determining step and making an approximation, it is possible to derive a rate expression for the reaction. For mechanism 1 , if the first step is rate determining, then the rate expression is just the rate expression for the first step. rate =k[I][S2O82] rate expression for mechanism 1a 0 2015 Cengage Leaming. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part. 332 If the second step is slower than the first, the rate expression is complicated and beyond the scope of this treatment. A second possible sequence is: 2I=I22I22+S2O82=I2(aq)+2SO42mechanism2 If the first step is the rate determining step: rate=2k[I]2rateexpressionformechanism2-a If the second step is the rate determining step, the rate expression is essentially the same as if the reaction occurred in one step. rate =k[I2[S2O82] rate expression for mechanism 2b and one step rxn. Your experimental results should enable you to distinguish among the three rate expressions and eliminate some of the possible mechanisms. Rate studies cannot prove that a proposed mechanism is operative but only disprove possible mechanisms. If you performed Experiment 25 , you observed that iodine reacts in the presence of iodide to form triiodide ion. This is a fast secondary reaction and was not included in the discussion above about the mechanism of the reaction. However, the triiodide formation is an extremely important part of the second method (spectroscopic) for following the reaction. METHOD 1 - Visual clock reaction. The solutions for each of the kinetic runs are given in the table on the next page. The sodium thiosulfate (Na2S2O3) is used as part of an indicator system (a purple color will form) to enable you to determine the time required for the reaction to occur for each kinetic run. As the amount of product formed in each case (SO42) will be the same, the relative rates of reaction will be inversely proportional to the reaction time. For example, a reaction that takes 25 seconds has twice the rate of one that takes 50 seconds. Because of the proportionality, for the analysis today, it will be possible to substitute the inverse of the time (1/t) for the actual rate of the reaction. To study the effect of reactant (Iand S2O82) concentrations on reaction rates, the reactant concentrations will be decreased. The simplest method for achieving this goal is to use a smaller amount of one of the reactants while keeping the total volume constant by replacement with water containing a substance that will not affect the reaction but that will maintain the same concentration of ions. The solutions of KCl and K2SO4 are used for this purpose. Experiment 27 A. Concentration effects on reactivn rates. For coverenience, five 50mi bureis shobld te cleanad and fillad with solutions a - e. If 5 groups of 2 stubents each set p core duret. considemble time should he suved. A dropper botile should be avaliable couciz of solusions a - d mo 125 or 250 -1. Erlenmeyer flask (for rum s also aba l dask Quickly add the contents of the Meacere the reyuired amoun of solutione ew begin timing and swirl watil thorough miving is achieved. Reourd the time elapsed until the puple color appears. For each rum after the purple B. Temperature effects on reaction rates. To study the effect of temperanure un we reaction rate, run 1 will be repeated at several temperatures. The flask containing solutions a and b will be heated or cooled and then solution e (at room temperature) will be added. It will be assumed that quick mixing will result in the same temperature for the whole solution. The temperature will be measured immediately after the purple color appears. For each run, add 20mL of solution a and 10mL of solution b to the first flask. Add 20mL. of solution e to the second flask. Change the temperature of the first flask to about the value indicated below. Quickly add solution e, commence timing and stir. Record the time elapsed for the purple to appear and measure the temperature of the solution. Repeat the procedure for the remaining runs. To raise the temperature of a flask, suspend the flask in a beaker of water and heat the beaker over a wire gauze with a Bunsen bumer. To lower the temperature of a flask, swirl the solution in an ice bath until the desired temperature is achieved. METHOD 2 - Spectroscopic Monitoring. The solutions for each of the kinetic runs are the same as for the Method 1 (except the sodium thiosulfate (Na2S2O3) solution is not needed). You will need a spectrometer capable of doing measurements down to at least 350nm and a couple of 334 cuvettes. You will also either need the uv absorption spectrum of triiodide or you will need to perform Experiment 25 - Part A to determine the optimal wavelength for this experiment. A. Concentration effects on reaction rates. It is necessary in this experiment to add and mix solutions quickly (especially the addition of KI ). To accomplish the speed needed, 1mL calibrated Beral pipets (calibrated at 0.25,0.50,0.75 and 1mL ) are ideally suited. The 3.00mL of water can be delivered with multiple transfers with a 1mL Beral pipe or better with a buret or a 3 mL pipet. Transfer all of the amounts except the KI into a cuvette and stir. Load another Beral pipet with the proper amount of KI solution, quickly squirt it into the cuvette, start a timer, mix thoroughly and quickly, and insert the cuvette into a precalibrated spectrometer. Measure the absorption starting at 10.0 seconds and then every 10.0 seconds up to 100 seconds. Withdraw the tul ...nwwa the tamnerature of the contents. The analysis of the rate data could in theory be performed using an integrated rate law. However, it is rather complex for this treatment and suffers from a significant figure problem. As the percent conversions are very small for these reactions, the method of initial rate analysis is valid and much easier to perform. All that is needed to determine the dependence of the reaction rate on the concentrations of iodide and persulfate is the relative rate of the initial reaction. To obtain this data, the absorption data is plotted vs time and the slope of the line is proportional to the rate. Use of a spreadsheet considerably facilitates this analysis. B. Temperature effects on reaction rates. To study the effect of temperature on the reaction rate, run 2 will be repeated at three temperatures. The data for room temperature from Part A above is applicable for run 2 ' below. Load the cuvette with all of the solutions except the KI solution. Cool the cuvette to a little below 10C and record the temperature. Squirt the KI solution into the cuvette, mix thoroughly and quickly, start the timer and insert the cuvette into the spectrometer. Take absorption measurements starting at the 10 second mark every 10 seconds up through 100 seconds. The temperature of the tube should be measured again immediately after removal of the tube from the spectrometer. The average of the temperatures recorded before and after the run will be taken as the temperature of the run. The process above should be repeated twice more with the tube preheated in a water bath to about 30C and 40C prior to the addition of the KI solution. Remember to read the temperature of the cuvette before and after each kinetic run. The absorptions are plotted versus time to determine the relative rates and then log10 (relative rate) is plotted versus the reciprocal of the average Kelvin temperature to obtain the activation energy. C 2015 Cengage Learning. All Rights Reserved. May not be scanned, copied or duplicated, or posted to a publicly accessible website, in whole or in part
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


