Write the equilibrium constant for the following reaction and calculate the value of K at 298 K
Question:
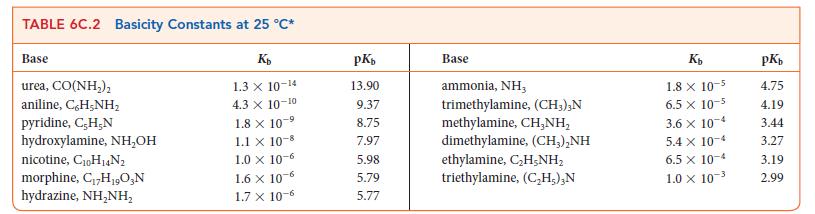
Write the equilibrium constant for the following reaction and calculate the value of K at 298 K for the reaction HNO2(aq) + NH3(aq) ⇌ NH4+ (aq) + NO2 – (aq) using the data in Tables 6C.1 and 6C.2.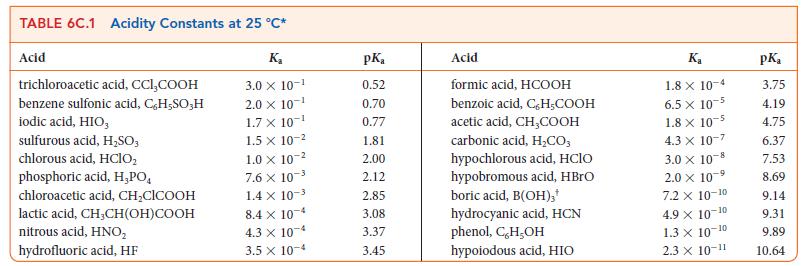
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H-SO;H iodic acid, HIO, sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H,PO chloroacetic acid, CH₂ClCOOH lactic acid, CH,CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF K₂ 3.0 X 10-¹ 2.0 × 10-¹ 1.7 X 10-¹ 1.5 x 10-² 1.0 x 10-² 7.6 X 10-³ 1.4 x 10-³ 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK₂ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C,H,COOH acetic acid, CH₂COOH carbonic acid, H₂CO3 hypochlorous acid, HClO hypobromous acid, HBrO boric acid, B(OH);* hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO Ka 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 × 10-8 2.0 x 10-5 -9 7.2 x 10-10 4.9 X 10-¹ -10 1.3 × 10-10 2.3 × 10-11 pK₂ 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
K KHN...View the full answer

Answered By

Vijesh J
My passion to become a tutor is a lifetime milestone. Being a finance and marketing professional with hands-on experience in wealth management, portfolio management, team handling and actively contributing in promoting the company. Highly talented in managing and educating students in most attractive ways were students get involved. I will always give perfection to my works. Time is the most important for the works and I provide every answer on time without a delay. I will proofread each and every work and will deliver a with more perfection.
4.70+
5+ Reviews
15+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the following exercises, show that matrix A is the inverse of matrix B. 1 2 _A = [ 3 4], B = -2 N/WN 3 2 1 2
-
Does the function f(x) appear to be continuous on the interval 0 x 2? If not, what about on the interval 0 x 0.5? 1 f(x) 1 x 2
-
Zumbrunn Company's income statement contained the following condensed information. ZUMBRUNN COMPANY Income Statement For the Year Ended December 31, 2020 Service revenue $970,500 Operating expenses,...
-
The par yield curve for U.S. Treasury bonds is currently flat across all maturities at 5.50 percent. You have observed following "paired" transaction by your bond portfolio manager: Bond G H...
-
Common costs. Wright Inc. and Brown Inc. are two small clothing companies that are considering leasing a dyeing machine together. The companies estimated that in order to meet production, Wright...
-
Development of a new deluxe version of a particular software product is being considered by Ravi Beharas software house. The activities necessary for the completion of this project are listed in the...
-
Climate Change In July 2015, a poll asked a random sample of 1,236 registered voters in Iowa whether they agree or disagree that the world needs to do more to combat climate change. The results show...
-
The following is the ending balances of accounts at December 31, 2018 for the Vosburgh Electronics Corporation. Additional Information: 1. The common stock represents 1 million shares of no par stock...
-
What strategies and tactics are employed to manage strategic risks and uncertainties, including geopolitical instability, supply chain disruptions, and emerging competitive threats, while preserving...
-
An aqueous solution is 35.0% by mass methylamine (CH 3 NH 2 ); its density is 0.85 g cm 3 . (a) Draw the Lewis structuresof a methylamine molecule and its conjugate acid. (b) If 80.0 mL of this...
-
A lead electrode in 0.020 m Pb(NO 3 ) 2 (aq) is connected to a hydrogen electrode in which the pressure of H 2 is 1.0 bar. If the cell potential is 0.078 V at 25C, what is the pH of the electrolyte...
-
OPoole Corporation develops and markets a harness in which a baby may be placed to bounce up and down. OPoole is considering approaching the bond market to raise funds to support the expansion of its...
-
You want to use a viscous Newtonian fluid to transport small granite particles through a horizontal $1 \mathrm{in}$. ID $100 \mathrm{ft}$ long pipeline. The granite particles have a diameter of $1.5...
-
When an analyst determines the value of a private company owned 100% by a single investor by analyzing the share prices of publicly traded companies, what adjustments must he or she make in order to...
-
Develop a public financing plan for a new NBA arena in the city of Sacramento. The arena will have a total cost of $350 million, and the public will finance 60% of the construction cost. Both the...
-
Suppose a community is considering constructing a large pool facility for use by community residents. How might it go about conducting a feasibility study for the pool? a. Describe possible methods...
-
You are tasked with calculating the property tax needed to fund construction and operation of a \($22.5\) million complex. The facilitys annual operating budget is forecast at \($3.6\) million, to be...
-
The Jogirushi Company makes a line of premium rice cookers, specially designed for the Japanese market. In February, Jogirushi launched a new model that is exclusively made in its Osaka factory. The...
-
Frontland Advertising creates, plans, and handles advertising campaigns in a three-state area. Recently, Frontland had to replace an inexperienced office worker in charge of bookkeeping because of...
-
Explain why colligative properties depend only on the concentration, and not on the identity of the molecule.
-
Ratcliffe and Chao [Canadian Journal of Chemical Engineering 47 (1969): 148] obtained the following tabulated results for the variation of the total pressure above a solution of isopropanol (P * 1 =...
-
At a given temperature, a nonideal solution of the volatile components A and B has a vapor pressure of 795 Torr. For this solution, y A = 0.375. In addition, x A = 0.310, P * A = 610. Torr, and P * B...
-
For example, a bank has assets worth IDR 100 billion and equity of IDR 10 billion. This bank's leverage ratio is 10. If the value of the bank's assets falls 5% to IDR 95 billion, the bank's equity...
-
2. Consider a portfolio that includes 10 private loans of $120,000 each and 200 bonds that costs $1500 each. Each private loan has a duration of 4 and convexity of 8.5 and each bond have a duration...
-
The estimated current purchasing price of a discount bond with a face value of $1500 and a yield to maturity of 9% is $_______ (Round your response to the nearest two decimal place) What is the...

Study smarter with the SolutionInn App


