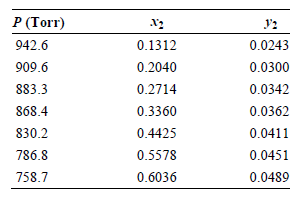
Ratcliffe and Chao [Canadian Journal of Chemical Engineering 47 (1969): 148] obtained the following tabulated results for
Question:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





