You are an environmental chemist studying a local waterway and need to know the amounts of all
Question:
You are an environmental chemist studying a local waterway and need to know the amounts of all forms of phosphate in the stream. You might begin by making up some solutions of phosphoric acid to use as standards. Calculate the concentrations of all solute species in 0.10 m H3PO4(aq).
ANTICIPATE Because successive deprotonations result in ever weaker acids, you should expect the concentrations to be in the order H3PO4 > H2PO4– > HPO4 2– > PO4 3–, with very, very little of the completely deprotonated species. Because the solution is acidic, you should also expect the concentration of OH– to be very small.
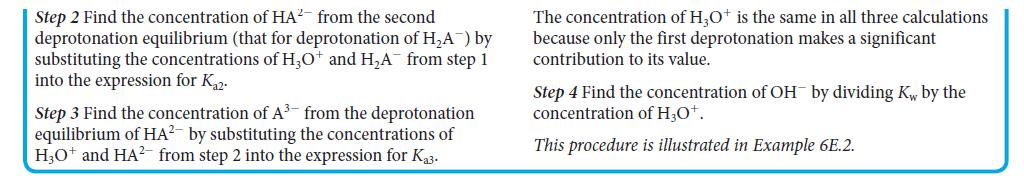
PLAN Follow the procedure for a triprotic acid in Toolbox 6E.1.
What should you assume? Assume that only the first deprotonation affects the pH and that the autoprotolysis of water has no significant effect on the pH.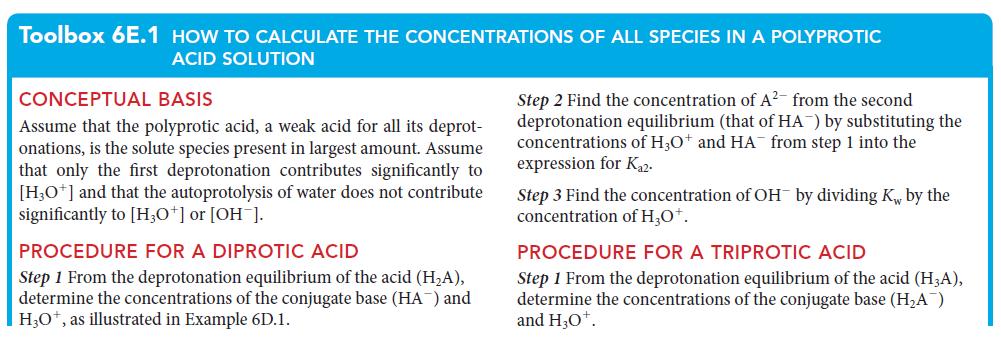
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





