You can imagine Rydbergs excitement, just after he had identified his formula and found that it worked
Question:
You can imagine Rydberg’s excitement, just after he had identified his formula and found that it worked for all the known lines in the spectrum of atomic hydrogen. Calculate the wavelength of the radiation emitted by a hydrogen atom for n1 = 2 and n2 = 3. Identify the spectral line in Fig. 1A.10b.
ANTICIPATE Because n1 = 2 the wavelength should match one of the lines in the Balmer series.
PLAN The frequency is given by Eq. 2. Convert frequency into wavelength by using Eq. 1.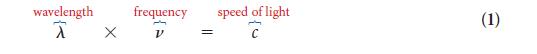
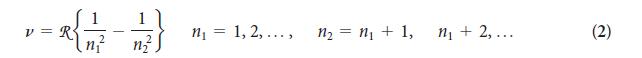
Fig. 1A.10b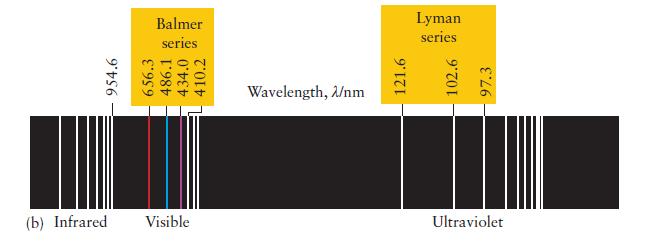
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





