You cant always guess a molecular shape from its chemical formula; even quite simple molecules can have
Question:
You can’t always guess a molecular shape from its chemical formula; even quite simple molecules can have surprising shapes. Predict the shape of a sulfur tetrafluoride molecule, SF4.
ANTICIPATE You might guess that SF4 has a tetrahedral geometry. However, you need to be aware that sulfur is capable of having an expanded valence shell. You need to follow the steps in Toolbox 2E.1 to predict the shape of SF4.
PLAN Use the procedure in Toolbox 2E.1.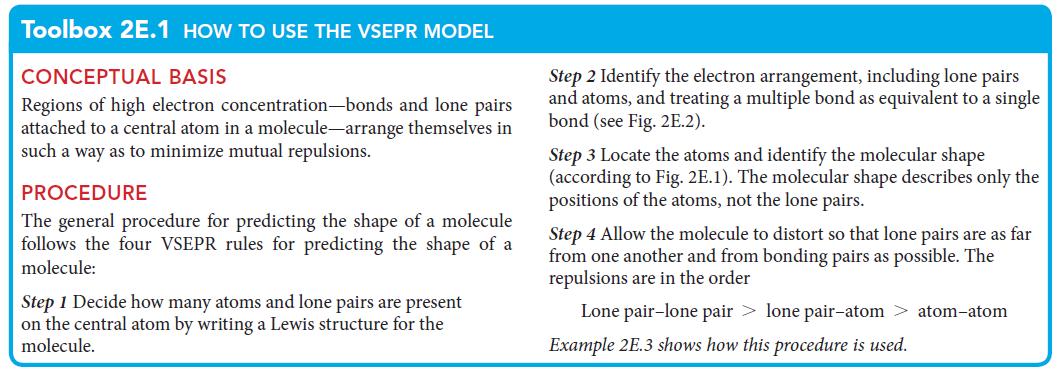
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





