You have made up a solution of known molarity but now realize that you need to know
Question:
You have made up a solution of known molarity but now realize that you need to know the molality instead. Find the molality of sucrose, C12H22O11, in 1.06 m C12H22O11(aq), which is known to have density 1.140 g · mL–1.
ANTICIPATE The mass of 1 L of aqueous solution is close to 1 kg, so the numerical value of the molality can be expected to be close to that of the molarity, but with different units, of course.
PLAN Use procedure 3 in Toolbox 5E.1, taking note of the discussion of units.
Transcribed Image Text:
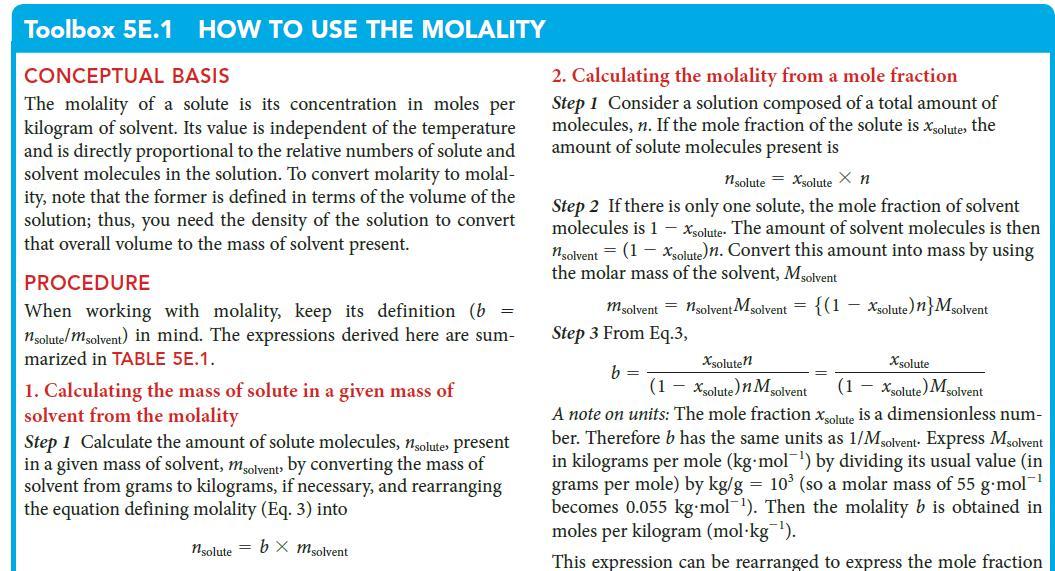
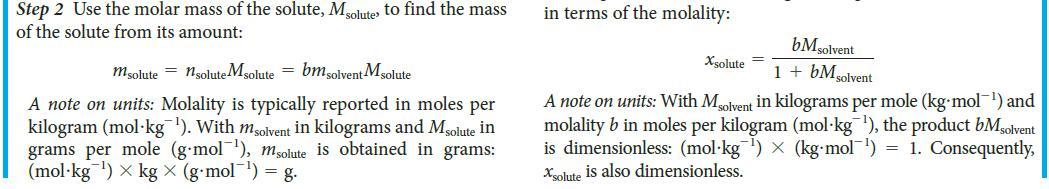
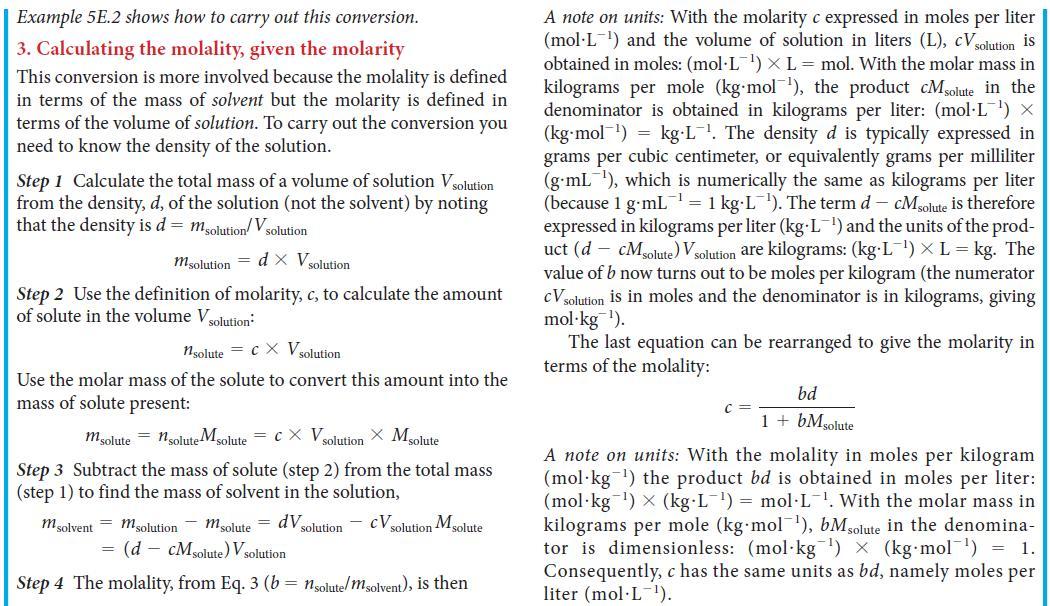
Toolbox 5E.1 HOW TO USE THE MOLALITY CONCEPTUAL BASIS The molality of a solute is its concentration in moles per kilogram of solvent. Its value is independent of the temperature and is directly proportional to the relative numbers of solute and solvent molecules in the solution. To convert molarity to molal- ity, note that the former is defined in terms of the volume of the solution; thus, you need the density of the solution to convert that overall volume to the mass of solvent present. PROCEDURE When working with molality, keep its definition (b = nsolute/msolvent) in mind. The expressions derived here are sum- marized in TABLE 5E.1. 1. Calculating the mass of solute in a given mass of solvent from the molality Step 1 Calculate the amount of solute molecules, nsolute present in a given mass of solvent, msolvent by converting the mass of solvent from grams to kilograms, if necessary, and rearranging the equation defining molality (Eq. 3) into nsolute = bx msolvent 2. Calculating the molality from a mole fraction Step 1 Consider a solution composed of a total amount of molecules, n. If the mole fraction of the solute is xsolute, the amount of solute molecules present is 1solute = Xsolute X n Step 2 If there is only one solute, the mole fraction of solvent molecules is 1 - Xsolute. The amount of solvent molecules is then nsolvent = (1 - Xsolute)n. Convert this amount into mass by using the molar mass of the solvent, Msolvent m solvent = nsolvent Msolvent = {(1 Step 3 From Eq.3, b = - Xsolute) n} Msolvent Xsolute Xsoluten (1-xsolute)n M solvent (1 - Xsolute) Msolvent A note on units: The mole fraction solute is a dimensionless num- ber. Therefore b has the same units as 1/Msolvent Express Msolvent in kilograms per mole (kg mol¹) by div its usual value (in grams per mole) by kg/g = 10³ (so a molar mass of 55 g-mol™¹ becomes 0.055 kg-mol¹). Then the molality b is obtained in moles per kilogram (mol kg ¹). This expression can be rearranged to express the mole fraction
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Note that a molar mass of 3423 gmol is equivalent to 03423 kgmol ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A colleague has been doing an experiment in which it was important to know the mole fractions of the components of a solution. You want to use the same solution, but you have in mind an experiment in...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
MiSTi, like many small technology companies, was born as an extension of the founder's special technical skills in the highly specialized field of "micro-switch" technology in the late 90's. Under...
-
State whether or not each of the following events would result in a liability being recognised in the accounts at 30 June. 1. Taxes for the year ended 30 June, which are not payable until October. 2....
-
You are asked to construct a parallel-plate, air-gap capacitor that will store 100 kJ of energy. (a) What minimum volume is required between the plates of the capacitor? (b) Suppose you have...
-
The essential problems of attempts to classify financial reporting practices across the world are related to the suitability of the data upon which such classifications have been based. Comment.
-
Review the information on YouTube in this section of the chapter and conduct your own research. What evidence is there that beginning with the end in mind was part of YouTubes founders original plan?
-
The file P10_67.xlsx contains hypothetical starting salaries for MBA students directly after graduation. The file also lists their years of experience prior to the MBA program and their class rank in...
-
I wanted a complete correct answer. Image transcription text AN procedure 2: Use formulas from AC 43-13.1B and reference information from the aircraft TCDS as necessary. For all adverse load checks,...
-
Given the following algorithm (4 points) Algorithm(B) for j2 to length [A] do key A[j] i- j - 1 while i > 0 and A[i] > key do A[i+1] A[i] ii-1 A[i+1] key (a) Describe the function of this algorithm...
-
The equilibrium constant for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g) is K = 2.5 * 10 10 at 500. K. Find the value of K for each of the following reactions at the same temperature. = NO(g) + O(g)...
-
Osmometry has been widely used in the polymer industry because it is a sensitive technique for determining the huge molar masses of polymer molecules. Now imagine that you are a polymer chemist; you...
-
Jane has been operating Mansfield Park as a C corporation and decides she would like to make an S election. What is the earliest the election will become effective under each of these alternative...
-
Find one solution for each of the difference equations below: (a) \(y(n)+2 y(n-1)+y(n-2)=0, y(0)=1\) and \(y(1)=0\) (b) \(y(n)+y(n-1)+2 y(n-2)=0, y(-1)=1\) and \(y(0)=1\).
-
If boys and girls are equally likely, groups of 400 births have a mean of 200 girls and a standard deviation of 10 girls. Is 232 girls in 400 births an unusually high number of girls?
-
Reconsider Problem 44 using an incremental present worth analysis. Data from problem 44 Dark Skies Observatory is considering several options to purchase a new deep-space telescope. Revenue would be...
-
Given a linear time-invariant system, prove the properties below: (a) A constant group delay is a necessary but not sufficient condition for the delay introduced by the system to a sinusoid to be...
-
We define the even and odd parts of a sequence \(x(n), \mathcal{E}\{x(n)\}\) and \(\mathcal{O}\{x(n)\}\) respectively, as \[\begin{aligned}\mathcal{E}\{x(n)\} & =\frac{x(n)+x(-n)}{2}...
-
Determine the break-even volume of work for a company with a fixed overhead of $72,000 and a contribution margin ratio of 14.0%.
-
Four GWU students have been selected to taste food sold by 3 different food trucks labeled as food truck A, B and C on H & 22nd Streets every Monday for 3-weeks. For each student, food trucks are...
-
Propose a stepwise mechanism for the following transformation. Et Me 1) Excess EtMgBr Me OH 2) H,0 'Et
-
Predict the products for each of the following: a. b. c. d. 1) Hg(OAc), 2) NABH, 1) Hg(OAc), e 2) NaBH,
-
Starting with benzene and using any other necessary reagents of your choice, design a synthesis for each of the following compounds. Each compound has a Br and one other substituent that we did not...
-
Air is compressed in a steady-state, steady-flow, adiabatic process from 0.1 MPa, 20C. During the compression process the temperature becomes 140C. If the mass flow rate is 0.2 kg/s, determine the...
-
A 0.07 m^3 rigid tank that is equipped with a stirring device initially contains 2kg of R-134a at a pressure of 800kPa. The system loses heat to the surroundings while 50kJ of work is applied to the...
-
Water initially at 200 kPa and 300C is contained in a piston-cylinder device fitted with stops. The water is allowed to cool until the piston rests on the stops where the volume is 0.673 of the...

Study smarter with the SolutionInn App


