The equilibrium constant for the reaction 2 NO(g) + O 2 (g) 2 NO 2 (g)
Question:
The equilibrium constant for the reaction 2 NO(g) + O2(g) ⇌ 2 NO2(g) is K = 2.5 * 1010 at 500. K. Find the value of K for each of the following reactions at the same temperature.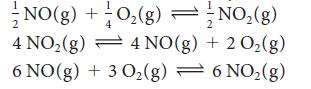
Transcribed Image Text:
= NO(g) + O₂(g) NO₂(g) 4 NO(g) + 2 O₂(g) 4 NO₂(g) 6 NO(g) + 3 0₂(g) = 6 NO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
Given the reaction 2NOg O2g 2NO2g with equilibrium constant K 25 1010 at 500 K we can find t...View the full answer

Answered By

Rehab Rahim
I am well versed in communicating and teaching in areas of all business subjects. I have helped many students in different ways from answering answers to writing their academic papers.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
The equilibrium constant for the reaction H2 + at 1 atm and 1500C is given to be K. Of the reactions given below, all at 1500C, the reaction that has a different equilibrium constant is (a) H2 + 12O2...
-
Find the equilibrium constant for the reaction 2NO + O2 2NO2 from the elementary reactions in Table A.10 to answer which of the nitrogen oxides, NO or NO2; is the more stable at...
-
In Exercises 2748, find the open intervals where the functions are concave upward or concave downward. Find any inflection points. f(x) = 2e x2
-
Consider two parallel-plate capacitors, C1 and C2, that are connected in parallel. The capacitors are identical except that C2 has a dielectric inserted between its plates. A voltage source of 200 V...
-
How do the causal factors discussed in the chapter affect corporate governance structures in different countries?
-
Show all the steps leading to the integral balance equation (18.6) in the text. Use the following boundary conditions and verify Eq. (18.7). (i) At \(y=0, T=T_{\mathrm{S}}\). (ii) At...
-
The following transactions were incurred by Howley Fabricators during January, the first month of its fiscal year. Requirements 1. Record the proper journal entry for each transaction. a. $ 180,000...
-
How do cognitive biases and cultural differences impact collaborative decision-making processes, and what techniques can be implemented to mitigate their effects in high-stakes environments ? Explain
-
After the success of the companys first two months, Santana Rey continues to operate Business Solutions. (Transactions for the first two months are described in the Chapter 2 serial problem.) The...
-
Which do you expect to have the higher vapor pressure at room temperature, octane, C 8 H 18 , or butane, C 4 H 10 ? Why?
-
You have made up a solution of known molarity but now realize that you need to know the molality instead. Find the molality of sucrose, C 12 H 22 O 11 , in 1.06 m C 12 H 22 O 11 (aq), which is known...
-
Draw the Haworth projection for the cyclic structure of D-mannose by laying down the Fischer projection.
-
On-Site Testing Service has received four investment proposals for consideration. Two of the proposals, X1 and X2, are mutually exclusive. The other two proposals, Y1 and Y2 are also mutually...
-
What percentage of women have red blood cell counts in the normal range from 4.2 to 5.4? Assume that red blood cell counts of women are normally distributed with a mean of 4.577 and a standard...
-
Suppose we have a system with transfer function having two zeros at \(z=0\), double poles at \(z=a\), and a single pole at \(z=-b\), where \(a>1\) and \(0
-
True or False: Present worth analysis is the most popular \(D C F\) measure of economic worth.
-
If boys and girls are equally likely, groups of 400 births have a mean of 200 girls and a standard deviation of 10 girls. Is 185 girls in 400 births an unusually low number of girls?
-
Determine the break-even volume of work for a company with a fixed overhead of $138,000, a contribution margin ratio of 8.9%, and a required level of profit of $100,000.
-
Chris Zulliger was a chef at the Plaza Restaurant in the Snowbird Ski Resort in Utah. The restaurant is located at the base of a mountain. As a chef for the Plaza, Zulliger was instructed by his...
-
Propose a plausible mechanism for each of the following transformations. a. b. c. d. e. f. OH 1) EtMgBr 2) H20
-
What product do you expect when tetrahydrofuran is heated in the presence of excess HBr?
-
Compound B has molecular formula C 6 H 10 O and does not possess any bonds. When treated with concentrated HBr, cis-1, 4-dibromocyclohexane is produced. Identify the structure of compound B.
-
Ps The traverse of end effector of Robot Manipulator is expressed as third degree polynomial function . The end effector joint has to move from an initial angle of 9 degrees to final angle of 49...
-
A flow of propane is compressed adiabatically from 100 kPa and 15C to 1.0 MPa. If the heats of mass are constant, what is the lowest temperature the propane can theoretically reach at the compressor...
-
One way to determine the quality of saturated steam is to throttle the steam to a low enough pressure that it exists as a superheated vapor. Saturated steam at 0.45 MPa is throttled to 0.1 MPa, 150C....

Study smarter with the SolutionInn App


