A commonly used equation in calorimetry analysis to determine the temperature at the maximum rate is given
Question:
A commonly used equation in calorimetry analysis to determine the temperature at the maximum rate is given by
\[ T_{\max }=\frac{E_{a}}{2 n R_{g}}\left[\sqrt{1+\frac{4 n R_{g} T_{F}}{E_{a}}}-1ight] \]
Derive this equation from Equation 8-21. Use this equation to calculate \(T_{\max }\) for the ethanol acetic anhydride system of Examples 8-3 and 8-4. Compare to the experimental value.
![xm 1 2n B -(T + 2n)+T [T+4n(1 + B)] (8-21)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1706/0/0/1/59965af84bfb3a9f1706001598683.jpg)

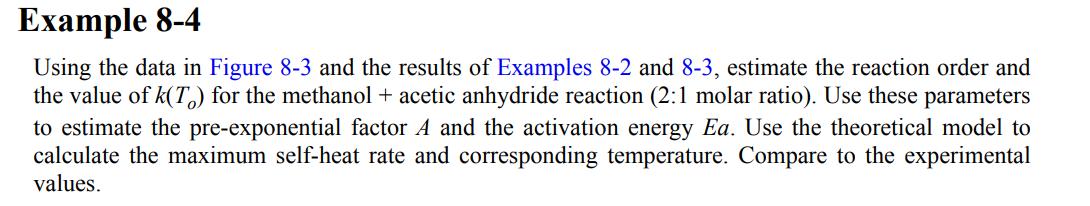
Figure 8-3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:





