Dilute aqueous ethanol (about 2-3%) is oxidized to acetic acid by the action of pure oxygen at
Question:
Dilute aqueous ethanol (about 2-3%) is oxidized to acetic acid by the action of pure oxygen at 10 atm in a trickle bed reactor packed with palladium-alumina catalyst pellets and kept at 30°C. According to Sato et al., Proc. First Pacific Chem. Eng. Congress, Kyoto, p. 197,1972, the reaction proceeds as follows: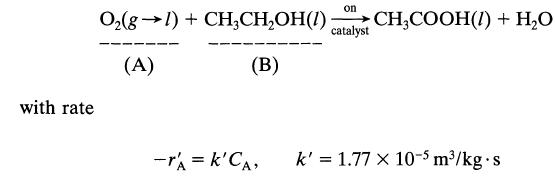
Find the fractional conversion of ethanol to acetic acid if gas and liquid are fed to the top of a reactor in the following system:

Transcribed Image Text:
with rate on catalyst O₂(g) + CH₂CH₂OH(1) CH3COOH(1) + H₂O (A) (B) -rA = k'CA, k' = 1.77 x 10-5 m³/kg-s
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
The material balance equations for the gas and liquid phases of a trickling bed reactor will be used to tackle this issue The kinetics of the reaction ...View the full answer

Answered By

Cyrus Sandoval
I a web and systems developer with a vast array of knowledge in many different front end and back end languages, responsive frameworks, databases, and best code practices. My objective is simply to be the best web developer that i can be and to contribute to the technology industry all that i know and i can do. My skills include:
- Front end languages: css, HTML, Javascript, XML
- Frameworks: Angular, Jquery, Bootstrap, Jasmine, Mocha
- Back End Languages: Java, Javascript, PHP,kotlin
- Databases: MySQL, PostegreSQL, Mongo, Cassandra
- Tools: Atom, Aptana, Eclipse, Android Studio, Notepad++, Netbeans.
Having a degree in Computer Science enabled me to deeply learn most of the things regarding programming, and i believe that my understanding of problem solving and complex algorithms are also skills that have and will continue to contribute to my overall success as a developer.
I’ve worked on countless freelance projects and have been involved with a handful of notable startups. Also while freelancing I was involved in doing other IT tasks requiring the use of computers from working with data, content creation and transcription.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Instead of using a trickle bed reactor for ethanol oxidation (see previous problem), let us consider using a slurry reactor. For this type of unit Take all flows and other values from the previous...
-
Ethyl alcohol can be bacterially oxidized to acetic acid in the following two-step fermentation sequence: 2 C2H5OH + O2 2 CH3CHO + 2 H2O 2 CH3CHO + O2 2 CH3COOH If the alcohol-containing feedstock...
-
Trickle bed reactors is widely used in the oil industry because of the advantages offered. In a trickle bed reactor, the oxidation of ethanol is to be carried out. The reaction is first order with...
-
In a survey of 1,002 people, 701 (or 70%) said that they voted in the last presidential election (based on data from ICR Research Group). The margin of error was 3 percentage points. However, actual...
-
What is marketing and what is its primary goal?
-
The advice about apples and ranges is not right. Consider the familiar equations for a circle C = 2r and A = r2. (a) Check that C and A have different dimensional formulas. (b) Produce an equation...
-
Assuming the short rate process of Exercise 2 and risk-neutral probabilities of .5 , consider a zero-coupon bond that pays \(\$ 1\) at time \(t=2\). Find the value at time \(t=0\) of this bond in two...
-
Refer to the statement of cash flows for both Kelloggs and General Mills for the most recent year and any other pertinent information reprinted at the back of this book. Required 1. Which method,...
-
2. A Corporation issues a bond with a face value of $250,000 and a stated rate of 8% interest. The interest is paid annually during the bond's 10-year life. The market rate for a similar bond is 6%....
-
FCC reactors are among the largest processing units used in the petroleum industry. Figure P1.3 shows an example of such units. A typical unit is 4-10 m ID and 10-20 m high and contains about 50 tons...
-
Predict the conversion of glucose to sorbitol in a stirred slurry reactor using pure hydrogen gas at 200 atm and 150C. The catalyst used is porous Raney nickel, and under these conditions Brahme and...
-
A random sample of five students is to be selected from 50 sociology majors for participation in a special program. a. In how many different ways can the sample be drawn? b. Show how the...
-
2. Draw a free body diagram for a. the massage therapist. Assume that ax = 0, ay = 0. (3) b. the client on the table. Assume that ax = 0, ay = 0 (3) c. the table. Assume that ax = 0, ay = 0 (3) 80 y X
-
Ia 5. Solve these two equations for a. mg sin 0-f=ma; f 2 r 6. Find in terms of from the given three equations. = fk-mg sin 0-0, N-mg cos 0=0, fk =N, Ans: k = tan 0 Ans: a = g sin 0 1+I/mr
-
2. Students perform a lab where they measure the relationship between drag and speed for an object of a given size and shape. The students use an electronic balance to determine the total mass of a...
-
2. Calculate the first derivative of the vector loop in the figure below, separate the Real and Imaginary components and simplify it as much as possible. Consider only angles 02, 03, 04 and vectors V...
-
At locations A and B, the electric potential has the values VA = 1.37 V and VB = 5.51 V, respectively. A proton released from rest at one of these locations passes through the other location. What is...
-
A firm has the option of borrowing $2.5 million through a 10-year loan with monthly payments based on a 7-percent lending rate, or entering into a 10-year, $2.5-million financial lease arrangement...
-
Write the binomial probability in words. Then, use a continuity correction to convert the binomial probability to a normal distribution probability. P(x 110)
-
A CSTR is being operated at steady state. The cell growth follows the Monod growth law without inhibition. The exiting substrate and cell concentrations are measured as a function of the volumetric...
-
Diabetes is a global epidemic affecting more than 240 million people worldwide. The vast majority of the cases are Type 2. Recently a drug, Januvia (J), was discovered to treat Type 2 diabetes. When...
-
In the summer of 2009, ExxonMobil decided to invest 600 million dollars on developing algae as an alternative fuel and their TV commercials on this initiative were recently shown (e.g., December...
-
If the estimated standard error of the mean for low income respondents is 0.26, the margin of error at a confidence level of 95% is _. Report the number rounded to the fourth decimal place (0.1234)...
-
6. (15 pts.) Here's a dataset I just generated. I'm not going to tell you what distribution I generated the data from because......I don't want to. 1.491, 0.019, 0.318, 0.056, 0.816, 0.978, 0.174,...
-
If the estimated standard error of the mean for low income respondents is 0.26, what is the upper limit of a confidence interval for a confidence level of 95%? Round to the second decimal place...

Study smarter with the SolutionInn App


