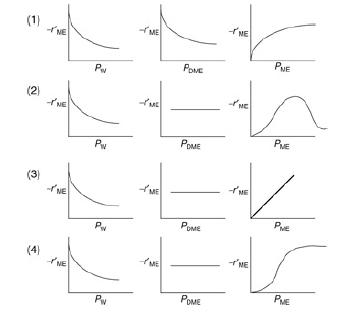
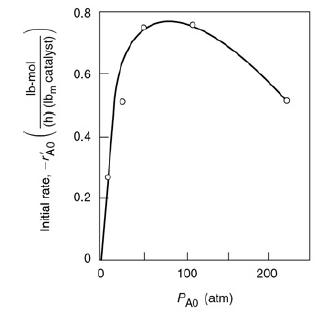
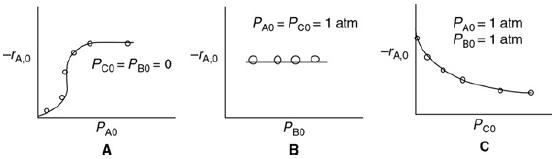
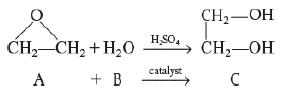
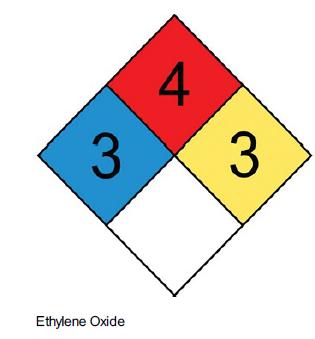
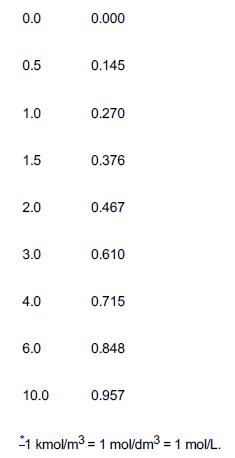
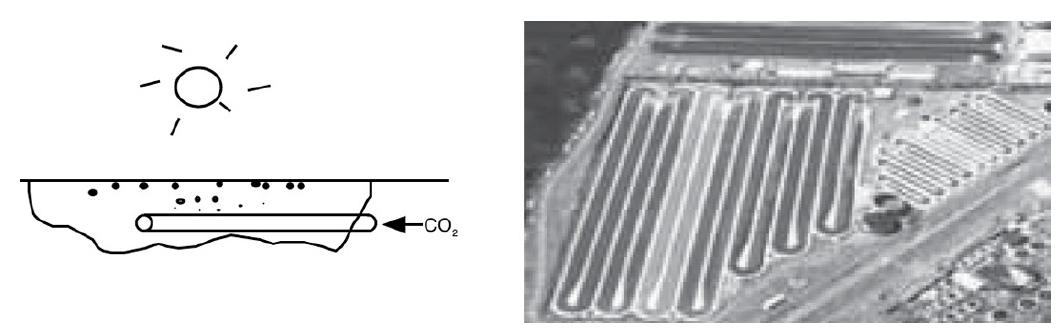
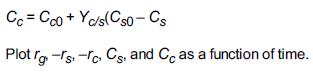Elements Of Chemical Reaction Engineering 6th Edition H. Fogler - Solutions
Discover the comprehensive solutions and answers key for "Elements Of Chemical Reaction Engineering 6th Edition" by H. Fogler. Access the online solution manual, complete with solved problems and chapter solutions, to enhance your understanding of complex concepts. Dive into the test bank for a thorough examination of key topics, and benefit from step-by-step answers provided in the instructor manual. This textbook's solutions PDF is perfect for anyone seeking a deeper comprehension of chemical reaction engineering. Explore questions and answers that facilitate learning, and enjoy the convenience of free download options to support your studies.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


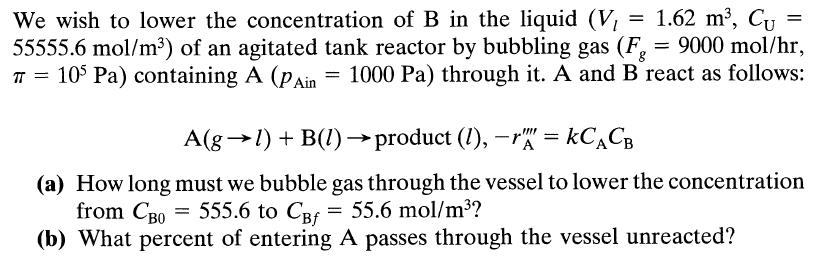
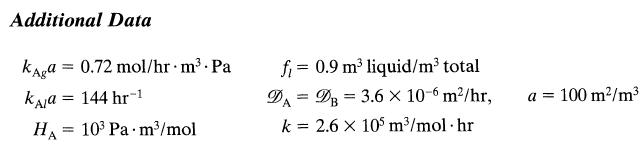
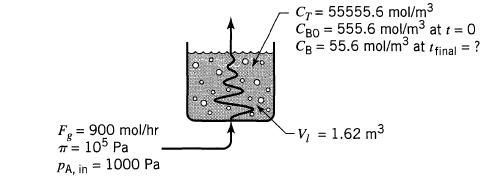
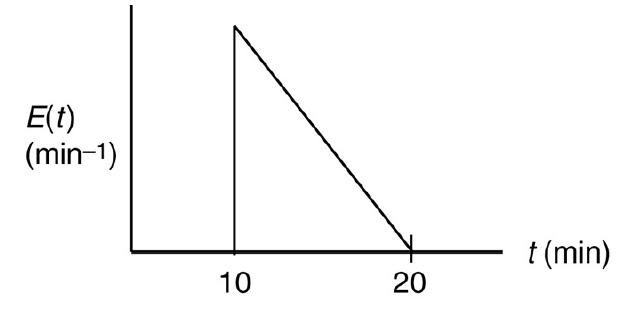


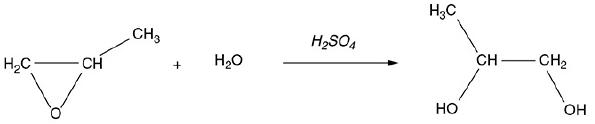
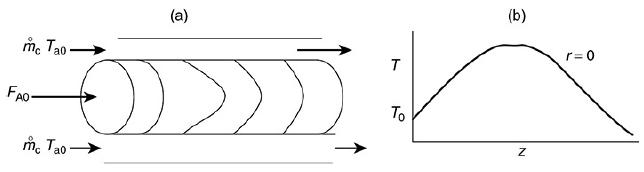
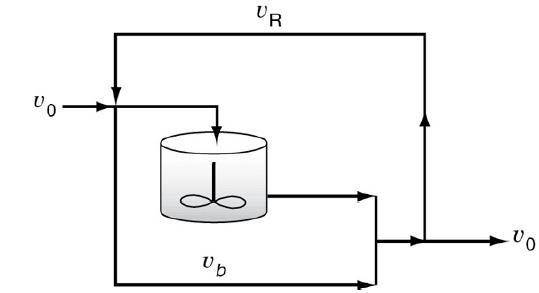
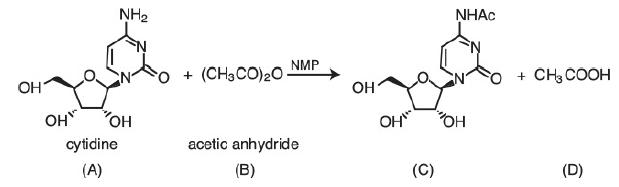
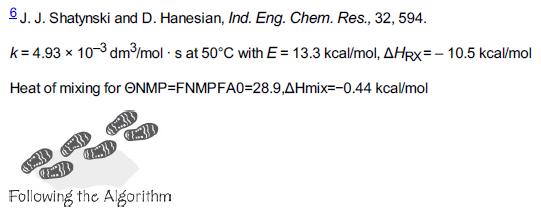
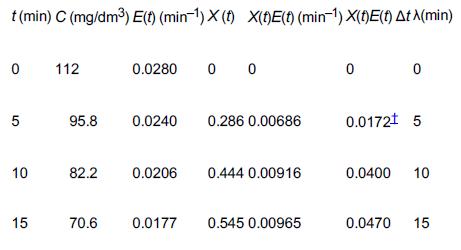
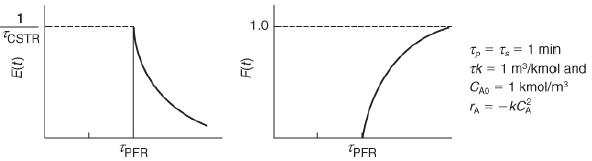

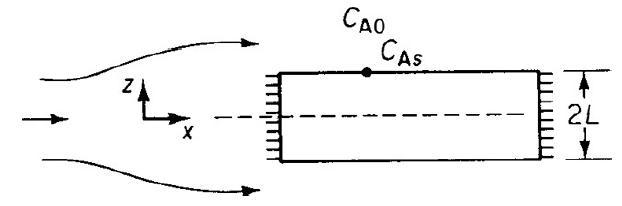

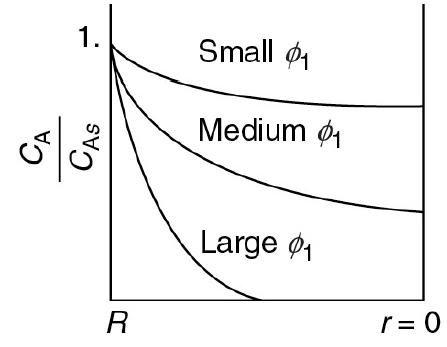
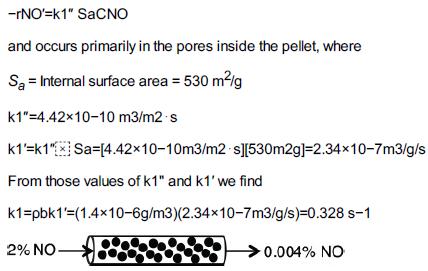


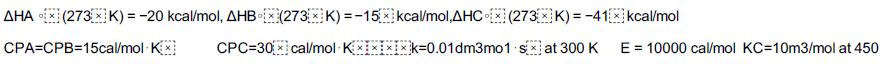


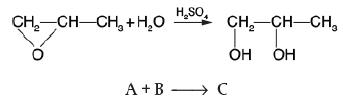
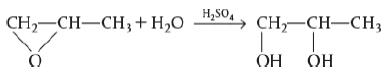
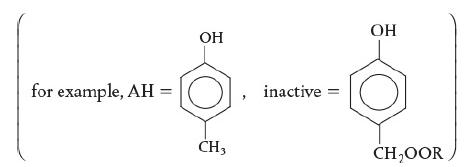
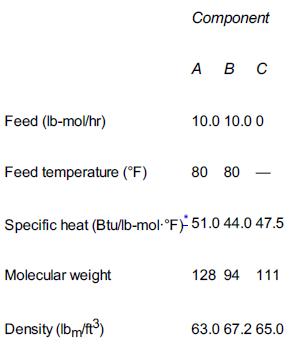
![HAO(273K)* =-20 kcal/mol,HBO(273 x K)-15 kcal/mol,HCo(273K) =-41x kcal/mol CPC=30x] cal/mol](https://s3.amazonaws.com/si.question.images/images/question_images/1697/6/1/1/687652f7fa71dfcb1697611685394.jpg)
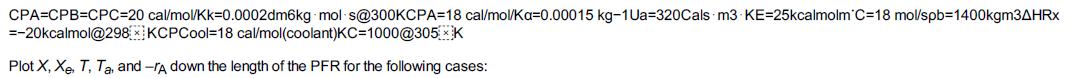


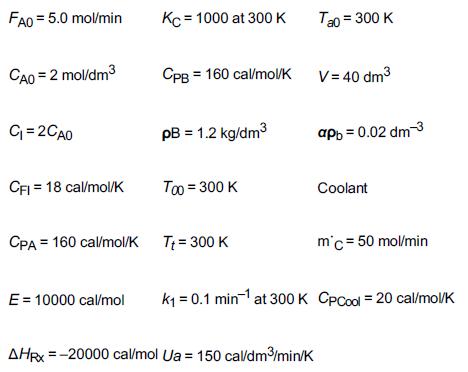
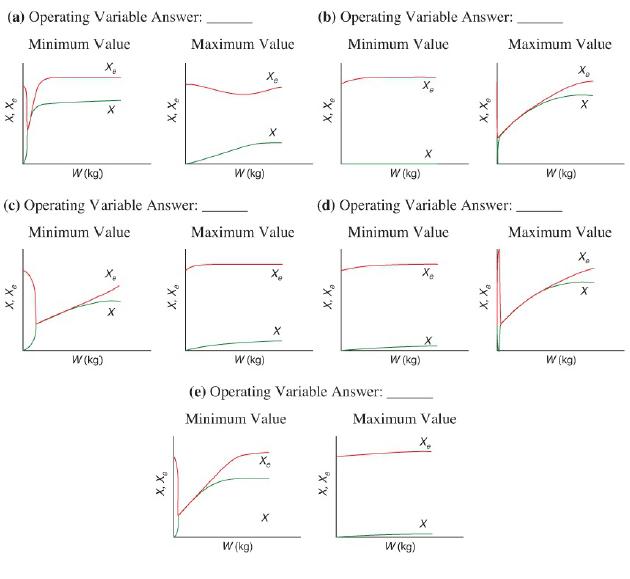



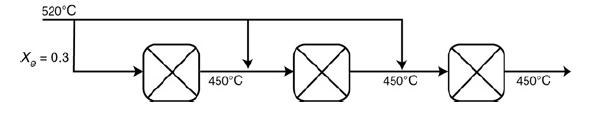
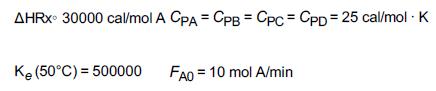
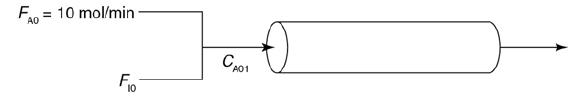


 and for the case ACp = 0 Ke=Ke2(T2)exp! [AHRx R(1T2-1T)](T11-2.4) 3.](https://s3.amazonaws.com/si.question.images/images/question_images/1697/5/1/8/659652e1443e222e1697518658619.jpg)