In catalytic dehydrogenation of hydrocarbons the catalyst activity decays with use because of carbon deposition on the
Question:
In catalytic dehydrogenation of hydrocarbons the catalyst activity decays with use because of carbon deposition on the active surfaces. Let us study this process in a specific system.
A gaseous feed (10% C4, H10, - 90% inerts, π = 1 atm, T = 555°C) flows (τ' = 1.1 kg · hr/m3) through a packed bed of alumina-chromia catalyst. The butane decomposes by a first-order reaction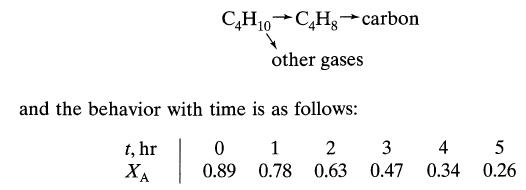
Examination of the 0.55-mm pellets shows the same extent of carbon deposition at the entrance and the exit of the reactor, suggesting concentration independent deactivation. Develop rate equations for reaction and deactivation. This problem was devised from the information given by Kunugita et al., J. Chem. Eng. (Japan), 2, 75 (1969).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





