Question: Repeat the above problem, except this time solve by the differential method. Data from above problem At room temperature sucrose is hydrolyzed by the catalytic
Repeat the above problem, except this time solve by the differential method.
Data from above problem
At room temperature sucrose is hydrolyzed by the catalytic action of the enzyme sucrase as follows: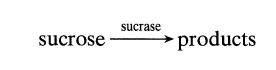
Starting with a sucrose concentration CA0 = 1.0 millimol/liter and an enzyme concentration CEO = 0.01 millimol/liter, the following kinetic data are obtained in a batch reactor (concentrations calculated from optical rotation measurements):

Determine whether these data can be reasonably fitted by a kinetic equation of the Michaelis-Menten type, or
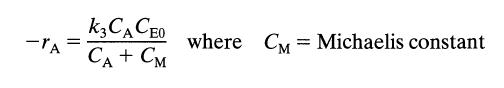
If the fit is reasonable, evaluate the constants k3 and CM. Solve by the integral method.
sucrose sucrase products
Step by Step Solution
3.28 Rating (145 Votes )
There are 3 Steps involved in it
To solve the problem using the differential method you will need to follow these steps Step 1 Calcul... View full answer

Get step-by-step solutions from verified subject matter experts


