Sophia and Nic are operating a batch reactor at their grandfathers plant in Krls, Jofostan. The reaction
Question:
Sophia and Nic are operating a batch reactor at their grandfather’s plant in Kärläs, Jofostan. The reaction is first-order, irreversible, liquid-phase, and exothermic. An inert coolant is added to the reaction mixture to control the temperature. The temperature is kept constant by varying the flow rate of the coolant (see Figure P13-4B).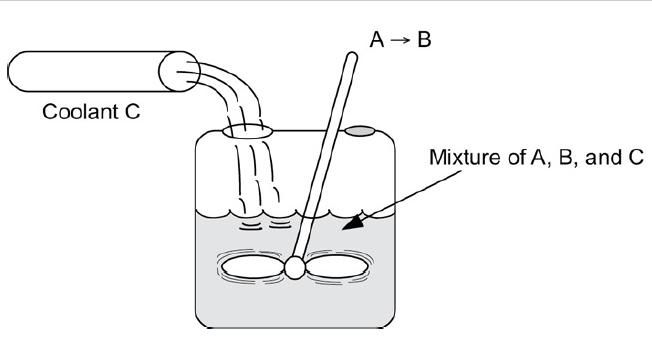
Semibatch reactor with inert coolant stream.
A reaction mixture of A and B is in the reactor to which a coolant C is fed making it a mixture of A, B, and C in order to maintain the temperature of the reactor. The stirrer is fully submerged in the mixture.
1. Help them calculate the flow rate of the coolant 2 hours after the start of the reaction.
2. It is proposed that rather than feeding a coolant to the reactor, a solvent be added that can be easily boiled off, even at moderate temperatures. The solvent has a heat of vaporization of 1000 Btu/lb and initially there are 25 lb-mol of A placed in the tank. The initial volume of solvent and reactant is 300 ft3. Determine the solvent evaporation rate as a function of time. What is the rate at the end of 2 hours?
Additional information: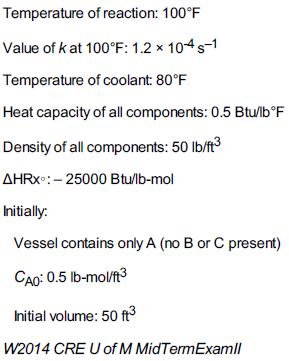
Step by Step Answer:






