Question: The equilibrium conversion is shown below as a function of catalyst weight down a PBR. The graph compares the values of conversion, X subscript e,
The equilibrium conversion is shown below as a function of catalyst weight down a PBR.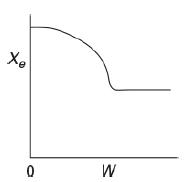
The graph compares the values of conversion, X subscript e, and weight W. Initially at W equals 0, the conversion is high. As the weight increases, the conversion gradually drops. The decrease stops at a certain weight, after which the conversion remains the same. Please indicate which of the following statements are true and which are false. Explain each case.
a. The reaction could be first-order endothermic and carried out adiabatically.
b. The reaction is first-order endothermic and the reactor is heated along its length with Ta being constant.
c. The reaction is second-order exothermic and cooled along the length of the reactor with Ta being constant.
d. The reaction is second-order exothermic and carried out adiabatically.
X 0 W
Step by Step Solution
3.38 Rating (160 Votes )
There are 3 Steps involved in it
a True Endothermic adiabatic so T decreases for endothermic reaction X e decrea... View full answer

Get step-by-step solutions from verified subject matter experts


