A 12.5-L scuba diving tank contains a heliumoxygen (heliox) mixture made up of 24.2 g of He
Question:
A 12.5-L scuba diving tank contains a helium–oxygen (heliox) mixture made up of 24.2 g of He and 4.32 g of O2 at 298 K. Calculate the mole fraction and partial pressure of each component in the mixture and the total pressure of the mixture.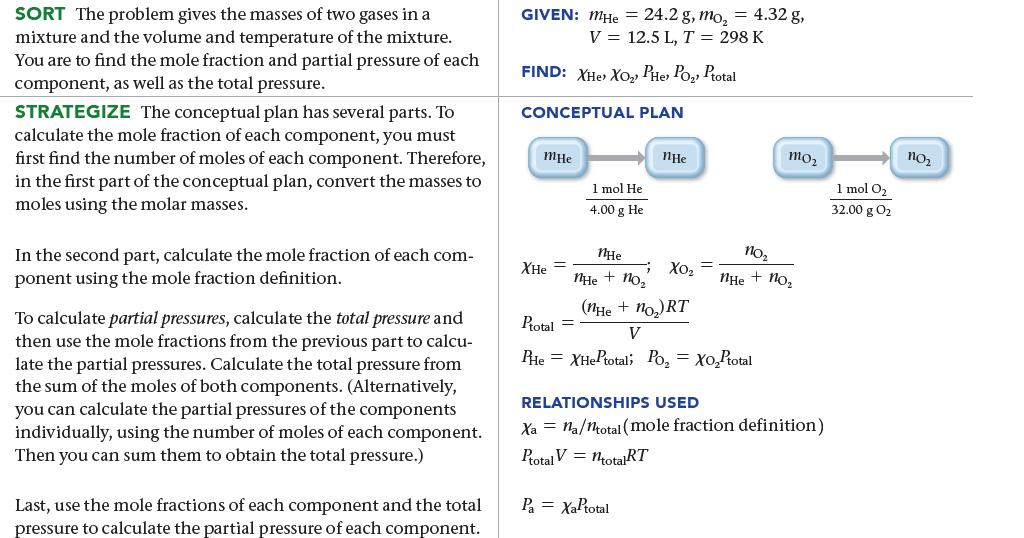
Transcribed Image Text:
SORT The problem gives the masses of two gases in a mixture and the volume and temperature of the mixture. You are to find the mole fraction and partial pressure of each component, as well as the total pressure. STRATEGIZE The conceptual plan has several parts. To calculate the mole fraction of each component, you must first find the number of moles of each component. Therefore, in the first part of the conceptual plan, convert the masses to moles using the molar masses. In the second part, calculate the mole fraction of each com- ponent using the mole fraction definition. To calculate partial pressures, calculate the total pressure and then use the mole fractions from the previous part to calcu- late the partial pressures. Calculate the total pressure from the sum of the moles of both components. (Alternatively, you can calculate the partial pressures of the components individually, using the number of moles of each component. Then you can sum them to obtain the total pressure.) Last, use the mole fractions of each component and the total pressure to calculate the partial pressure of each component. GIVEN: MHe = 24.2 g, mo₂ = 4.32 g, V = 12.5 L, T = 298 K FIND: XHe> XO₂ PHe› Po₂) Ptotal CONCEPTUAL PLAN mHe 1 mol He 4.00 g He XHe nHe 9 XO₂ nHe nHe + no₂ (nHe + no₂) RT Ptotal = V PHe XHEPtotal; Po₂ = XO₂Ptotal Pa = XaPtotal mo₂ no₂ nHe + no₂ RELATIONSHIPS USED Xa na/ntotal (mole fraction definition) Ptotal V = totalRT 1 mol O₂ 32.00 g 0₂ no₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
242 g He x 432 g 0 x XHe XO Ptotal 1 mol He 400 g He 1 mol O 3200 g ...View the full answer

Answered By

Shristi Singh
A freshman year metallurgy and material science student in India.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A solution contains 3.95 g of carbon disulfide (CS 2 ) and 2.43 g of acetone (CH 3 COCH 3 ). At 35 C the vapor pressures of pure carbon disulfide and pure acetone are 515 torr and 332 torr,...
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
Calculate the mole fraction of each component and the gas constant of the mixture for each of the following mixtures: a) 4 kg N2, 1 kg O2, 3 kg CO2 b) 4 kg N2, 1 kg CH2, 3 kg NH3 c) 5 kg air, 3 kg...
-
A decline in the aggregate demand has led to a decline in output in the economy equal to $10 billion. Government wants to counter this recession by reducing income taxes. Assuming that the marginal...
-
Describe some important uses of electronic commerce and explain why it is important to accountants.
-
Jurvin Enterprises recorded the following transactions for the just completed month. The company had no beginning inventories. a. $94,000 in raw materials were purchased for cash. b. $89,000 in raw...
-
The codon change (Gly-12 to Val-12) in human H-ras that converts it to oncogenic H-ras has been associated with many types of cancers. For this reason, researchers would like to develop drugs to...
-
The chart of accounts of LR Company includes the following selected accounts. 112 Accounts Receivable ......401 Sales Revenue 120 Inventory ...........412 Sales Returns and Allowances 126 Supplies...
-
Oil having a density of 921 kg/m floats on water. A rectangular block of wood 4.41 cm high and with a density of 963 kg/m floats partly in the oil and partly in the water. The oil completely covers...
-
Aluminum reacts with chlorine gas to form aluminum chloride. What minimum volume of chlorine gas (at 298 K and 225 mmHg) is required to completely react with 7.85 g of aluminum? a) 36.0 L b) 24.0 L...
-
The graph shows PV/RT for carbon dioxide at three different temperatures. Rank the curves in order of increasing temperature. (a) C (b) A (c) B (d) C PV/RT 2. 1.6- 1.2- 0.8- 0.4- 0- 0 200 400 600...
-
Blackfeather Tours sells scuba diving and kayaking excursions, along with a number of unique sightseeing packages. The company requires a 50% payment from the customer at the time of booking. The...
-
If Steve Pierce depreciated his truck by the double declining-balance method, calculate the depreciation expense for year 1. Cost Residual value Service of useful life $7,100 $2,400 4 years
-
What are the main ways users can access information stored in a DBMS?
-
What three roles can an accountant fill in relation to the AIS? Describe them.
-
Reggie Company on July 1, 201X, had inventory costing $31,500 and during July had net purchases of $67,200. Over the years, Reggie Companys gross profit averaged 43% on sales. Given that the company...
-
Describe CPA WebTrust.
-
Dexter Construction Corporation is building a student condominium complex; it started on January 1, Year 1. Dexter borrowed $2.5 million on January 1 specifically for the project by issuing a 10%,...
-
If someone's Z-score for a variable was 0.67. Their score is a significant extreme score. Their score is not significant. O Their score is slightly above average. O Their score is an outlier.
-
Determine the moments at D and C, then draw the moment diagram for each member of the frame. Assume the supports at A and B are pins and D and C are fixed joints. EI is constant. 5 k/ft 12 ft- 9 ft A
-
Determine the reactions at A and D. Assume the supports at A and D are fixed and B and C are fixed connected. EI is constant. 8k/ft 15 ft -24 ft-
-
Determine the moments at the ends of each member of the frame. Assume the joint at B is fixed, C is pinned, and A is fixed. The moment of inertia of each member is listed in the figure. E = 29(10 3 )...
-
Below are FIVE separate situations an auditor is faced with: Required (a) You have been approached by a prospective new audit client to undertake their audit. You request their permission to contact...
-
1. Briefly describe how a composite growth rate of demand for hotel rooms can be calculated. 2. In estimating total sales revenue for a coffee shop in a proposed new hotel, why is it important to...
-
In what way might a policy to pay no dividends affect a hotel corporations market price of shares? If the policy were to pay out all net income in dividends, how might this affect the company's...

Study smarter with the SolutionInn App


