A city of 100,000 people uses approximately 1.0 * 10 11 kJ of energy per day. Suppose
Question:
A city of 100,000 people uses approximately 1.0 * 1011 kJ of energy per day. Suppose all of that energy comes from the combustion of liquid octane (C8H18) to form gaseous water and gaseous carbon dioxide. Use standard enthalpies of formation to calculate ΔH°rxn for the combustion of octane and then determine the number of kilograms of octane necessary to provide this amount of energy.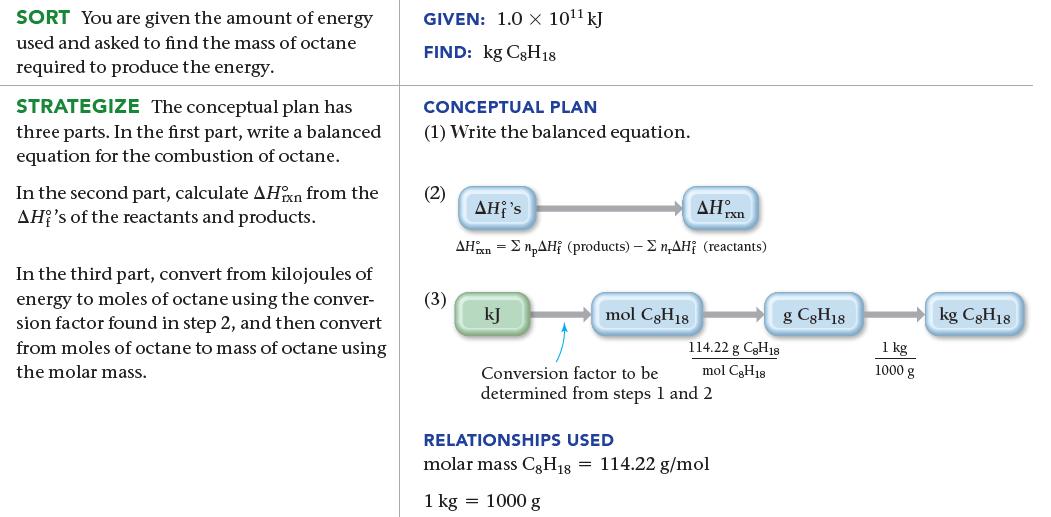
Transcribed Image Text:
SORT You are given the amount of energy used and asked to find the mass of octane required to produce the energy. STRATEGIZE The conceptual plan has three parts. In the first part, write a balanced equation for the combustion of octane. In the second part, calculate AHin from the AH's of the reactants and products. In the third part, convert from kilojoules of energy to moles of octane using the conver- sion factor found in step 2, and then convert from moles of octane to mass of octane using the molar mass. GIVEN: 1.0 x 10¹¹ kJ FIND: kg C8H18 CONCEPTUAL PLAN (1) Write the balanced equation. (2) (3) AHx AHi's AHxn npAH (products) - Σn,AH; (reactants) kJ mol C8H18 114.22 g C8H18 mol CH18 Conversion factor to be determined from steps 1 and 2 RELATIONSHIPS USED molar mass C8H18 = 114.22 g/mol 1 kg = 1000 g g C8H18 1 kg 1000 g kg C8H18
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
1 mol C8H18 50741 kJ ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Let the two primes p= 41 and q = 17 be given as set-up parameters for RSA. 1. Which of the parameters e= 32,e2 = 49 is a valid RSA exponent? Justify your choice. 2. Compute the corresponding private...
-
A psychologist conducts an experiment on rats. She puts a rat in a cage having three rooms labeled 1, 2, and 3, as shown in Figure below. L Figure: a cage with 3 rooms and 5 doors. The rats are...
-
Melissa Ostwerk, the new controller of TurboDrives, Inc., has just returned from a seminar on the choice of the activity level in the predetermined overhead rate. Even though the subject did not...
-
Figure shows nine graphs of position, velocity, and acceleration for objects in linear motion. Indicate the graphs that meet the following conditions: (a) Velocity is constant. (b) Velocity has...
-
Political expectations, inflation, and unemployment Consider a country with two political parties, Democrats and Republicans. Democrats care more about unemployment than Republicans, and Republicans...
-
For each of the following procedures taken from the quality control manual of a CPA firm, identify the applicable element of quality control from Table 2-4. a. Appropriate accounting and auditing...
-
Using financial statements for each of ROSS STORES and SUNOCO, which are provided to you on CANVAS: for the main year of reporting and the immediate preceding year, and using the latest version of...
-
One way to evaluate fuels with respect to global warming is to determine how much heat they release during combustion relative to how much CO 2 they produce. The greater the heat relative to the...
-
Which process is endothermic? a) The evaporation of water from the skin b) The burning of candle wax c) The oxidation of iron in a chemical hand warmer d) The combustion of natural gas in a stove
-
Examine the similarities and dissimilarities between CBT and RET.
-
Suppose that Glitter Gulch, a gold mining firm, increased its sales revenues on newly mined gold from $100 million to $200 million between one year and the next. Assuming that the price of gold...
-
On the basis of your answer to question 76, do you believe the advertisement is accurate? Question 76 A natural food company is marketing a new yogurt that it advertises as having only half the fat...
-
If in some country personal consumption expenditures in a specific year are $50 billion, purchases of stocks and bonds are $30 billion, net exports are $10 billion, government purchases are $20...
-
The company in question 76 further claims that only 2 % of the cups contain more than half the fat of regular yogurt. What is the probability of our seeing more than 12 cups out of 400 (which is what...
-
Assume that a grower of flower bulbs sells its annual output of bulbs to an Internet retailer for $70,000. The retailer, in turn, brings in $160,000 from selling the bulbs directly to final...
-
Wheeler Company began 2010 with 10,000 shares of $10 par common stock and 2,000 shares of 9.4%, $100 par, convertible preferred stock outstanding. On April 2 and June 1, respectively, the company...
-
Suppose you won a financial literacy competition and are given FJS10000 to invest, with the condition that investment can be done either in, i) Invest in Unit trust of Fiji or Invest in Fijian...
-
The A-36 steel pipe is subjected to the axial loading of 60 kN. Determine the change in volume of the material after the load is applied. 30 mm 40 mm 60 kIN 60 kN 0.5 m
-
Air is pumped into the steel thin-walled pressure vessel at C. If the ends of the vessel are closed using two pistons connected by a rod AB, determine the increase in the diameter of the pressure...
-
Determine the increase in the diameter of the pressure vessel in Prob. 1053 if the pistons are replaced by walls connected to the ends of the vessel. Problem: 10-53 Air is pumped into the steel...
-
Canyon Dental Services is a specialized dental practice whose only service is filling cavities. Canyon has recorded the following for the past nine months: Number of Cavities Month January Filled...
-
Scenario: You are working for a company that is hoping to adapt a short story into a film. Your supervising director had not read "The Happy Prince" by Oscar Wilde, and since she doesn't have time to...
-
Use the continuous compound interest formula to find the indicated value. a= 6100, r= 8.48% t= 8 years p=___ round to two decimal places Use the continuous compound interest formula to find the...

Study smarter with the SolutionInn App


