Based on these molecular views, determine whether each pictured acid is weak or strong. H3O+- HF- F
Question:
Based on these molecular views, determine whether each pictured acid is weak or strong.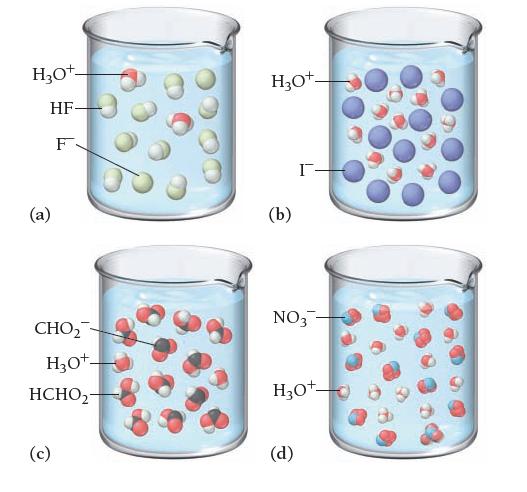
Transcribed Image Text:
H3O+- HF- F (a) CHO₂ H3O+- HCHO2- (c) H₂O+- (b) I- NO3- H₂O+ (d)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Weak ...View the full answer

Answered By

Michael Mulupi
I am honest,hardworking, and determined writer
4.70+
72+ Reviews
157+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Based on these molecular views, determine whether each pictured base is weak or strong. NH3- NH4+ (a) H2CO3- - Nat- HCO3- (c) OH Nat- (b) OH- Sr2+_ (d)
-
Disc jockey Lee Jason Kibler uses turntables and other performers' vocals to produce music containing jazz and funk elements. Since 1999, he has performed and released several albums under the name...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Describe the three ways a client can reference a name from a namespace in C++.
-
What is the cause of an unfavorable volume variance? Does the volume variance convey any meaningful information to managers?
-
The annual report for Colgate-Palmolive contains the following information (in millions): Income Taxes Temporary differences between accounting for financial statement purposes and accounting for tax...
-
When does a party have an automatic right to amend a pleading?
-
Analyzing and Interpreting the Financial Statement Effects of LIFO and FIFO Element Company uses a periodic inventory system. At the end of the annual accounting period, December 31, 2012, the...
-
Entries and Schedules for Unfinished Jobs and Completed Jobs Kaymer Industries Inc. uses a job order cost system. The following data summarize the operations related to production for January, the...
-
The binding of oxygen by hemoglobin in the blood involves the equilibrium reaction: In this equation, Hb is hemoglobin. The pH of normal human blood is highly controlled within a range of 7.35 to...
-
Identify the Lewis acid and Lewis base from among the reactants in each equation. a. Ag (aq) + 2NH3(aq) Ag(NH3)2+(aq) b. AlBr3 + NH3 H3NAIBr3 C. F (aq) + BF3(aq) = BF4 (aq)
-
Calculate the value for each of Exercises. 9 (8)
-
Consider the following series of future cash flows CF 5x Sx $350 5480 5675 5800 The present value for the entire cash flow series is $1,300. The discount rate is 9% Required: Estimate the value of...
-
Finding Future Value Looking for the spending power of these amount in 45 years How much will $1,000,000.00 be worth in 2066? How much will $53,000 be worth in 2066?
-
a.) Draw the Fx and FV Components b.) solve for Fx and Fy
-
Give reason for your answer A stone can slide down one of three different frictionless ramps, as shown in the figure. For which ramp will the speed of the ball be the greatest at the bottom? points...
-
Structured Pty. Ltd., a small business entity, has prepared the following income statement for 2017/18: Income $ Unfranked Dividends 40,000 Fully Franked Dividends (related franking credit of...
-
Who would use Altmans Z score to predict bankruptcy? Why would the ability to predict bankruptcy be useful to them?
-
In Problem 8.43, determine the smallest value of for which the rod will not fall out of the pipe. IA -3 in.-
-
Saturn makes one complete orbit of the Sun every 29.4 years. Calculate the radius of the orbit of Saturn.
-
In Section 5.4, we showed that the radius of a geosynchronous orbit about the Earth is 4.2 107 m, compared with the radius of the Earth, which is 6.4 106 m. By what factor is the force of gravity...
-
You are an astronaut (m = 95 kg) and travel to a planet that is the same radius and mass as the Earth, but it has a rotational period of only 2 h. What is your apparent weight at the equator of this...
-
1. Write a method that takes a string as input and prints true if the String length is greater than 6 characters. The method should print false otherwise. 2. Write a method that takes a String s and...
-
The analysis algorithm is known as follows: function Analysis(n:integer) ->integer {Initial State: n >= 0 Final State: Return calculation n(n+1)/2} Dictionary i: integer res: real Algorithm Res
-
The data has been collected from Form 5 secondary school students as a respondent to find out their confidence level in getting high marks for subject Additional Mathematics. The survey has been...

Study smarter with the SolutionInn App


