Based on what you learned in Chapter 2 about atoms, what part of the atom do you
Question:
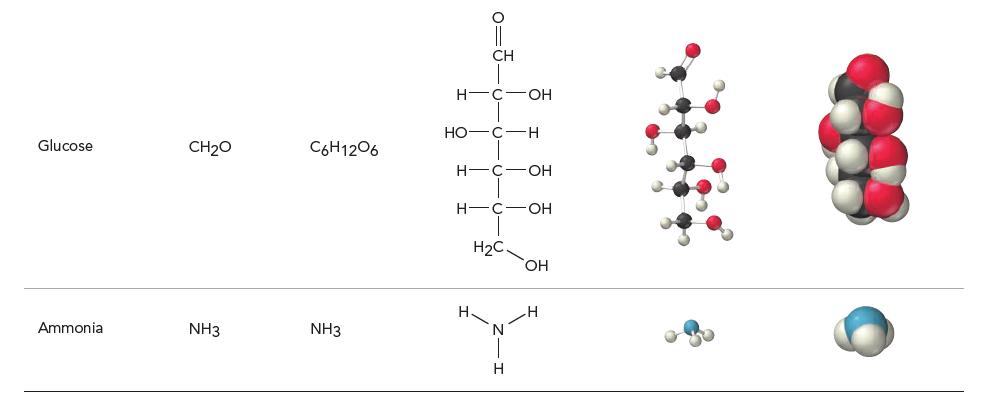
Based on what you learned in Chapter 2 about atoms, what part of the atom do you think the spheres in the molecular space-filling models shown in Table 3.1 represent? If you were to superimpose a nucleus on one of these spheres, how big would you draw it?
(a) Each sphere represents the hard outer shell of an atom. The nucleus would be too small to see on the same scale.
(b) Each sphere represents the electron cloud of the atom. The nucleus would be too small to see on the same scale.
(c) Each sphere represents the nucleus of an atom. The nucleus is the same size as the sphere.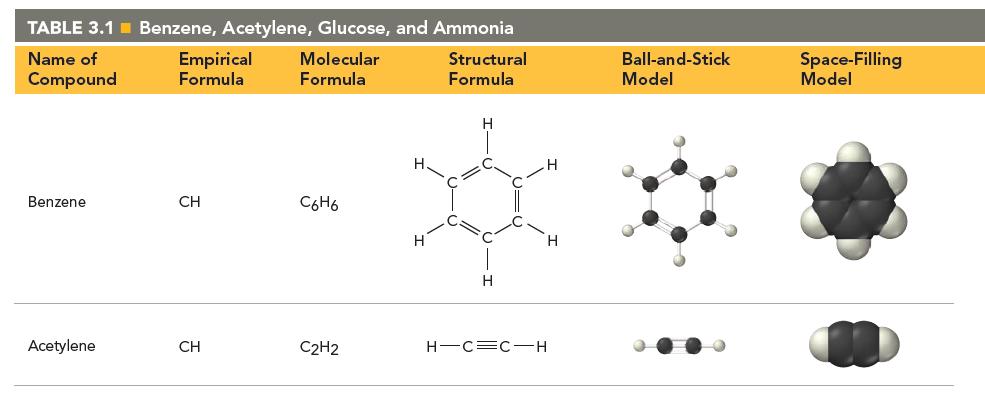
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





