Calculate H rxn for the reaction: Use the following reactions and given Hs: 5 C(s) + 6
Question:
Calculate ΔHrxn for the reaction:![]()
Use the following reactions and given ΔH’s: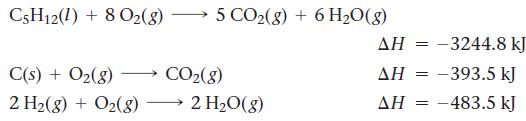
Transcribed Image Text:
5 C(s) + 6 H₂(8) C5H12(1)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)

Answered By

Sultan Ghulam Dastgir
The following are details of my Areas of Effectiveness English Language Proficiency, Organization Behavior , consumer Behavior and Marketing, Communication, Applied Statistics, Research Methods , Cognitive & Affective Processes, Cognitive & Affective Processes, Data Analysis in Research, Human Resources Management ,Research Project,
Social Psychology, Personality Psychology, Introduction to Applied Areas of Psychology,
Behavioral Neurosdence , Historical and Contemporary Issues in Psychology, Measurement in Psychology, experimental Psychology,
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
What are the advantages and disadvantages of organizing a business as a corporation? 2. How does a partnership differ from a limited liability company? 3. Why do corporates file for bankruptcy? 4....
-
Calculate S values for the following reactions by using tabulated values from Appendix C. In each case explain the sign of S. (c) HNO3(g) NH3 (g)- NH4NO3(s) 2 Fe203(s)4 Fe(s) 302(g) CaCO3(s,calcite)...
-
Smart Sets manufactures headphone cases. During September 2016, the company produced 108,000 cases and recorded the following cost data: Requirements 1. Compute the cost and efficiency variances for...
-
At Ratliff Company checks are not prenumbered because both the purchasing agent and the treasurer are authorized to issue checks. Each signer has access to unissued checks kept in an unlocked file...
-
A cable of length \(l\) and mass \(ho\) per unit length is stretched under a tension \(P\). One end of the cable is fixed and the other end is connected to a pin, which can move in a frictionless...
-
How and why does an auditor test purchases cutoff?
-
Peralta Company borrows $60,000 on July 1 from the bank by signing a $60,000, 10%, one-year note payable. (a) Prepare the journal entry to record the proceeds of the note. (b) Prepare the journal...
-
1.Taxpayer, a cash method, calendar year taxpayer, engaged in the following transactions in shares of stock. Consider the amount and character of T's gain or loss in each transaction: (a)T bought 100...
-
According to the published reports, practice under fatigued conditions distorts mechanisms which govern performance. An experiment was conducted using 15 college males who were trained to make a...
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: FeO3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(8)
-
Consider the generic reaction: Determine the value of H for each related reaction. A + 2B C + 3D AH = 155 kJ
-
Give an example of circumstances where an employee commits a tort but his employer is not liable. What element is necessary to make the employer liable?
-
Amanda Manufacturing Company prepared the following static budget income statement: Revenues $ 140,250.00 Variable Costs (85,250.00) Contribution Margin 55,000.00 Fixed Costs (32,000.00) Net Income $...
-
Private Equity Associates ("Associates") is a limited partnership that buys, restructures, and then sells companies that are not publicly traded. Manager, the sole general partner of Associates, is...
-
A stock just paid a dividend of $1.00. Its dividends are paid annually and are expected to grow 6.00% per year. The stock is trading for $26.50 today. What is the forward dividend yield (D1 / P) on...
-
The short-term financing policies can be flexible or restrictive. Discuss THREE (3) advantages and TWO (2) disadvantages of the Restrictive policy.
-
a) Discuss the objective of corporate financial manager. b) Explain why profit maximisation fails to be consistent with wealth maximisation. Include reasons why profit maximisation and/or wealth...
-
Design a management control framework for a company of your choice, based on social, environmental, and ethics indicators for the product or service offered. You can use Figure 14.17, The Process of...
-
An example of prescriptive analytics is when an action is recommended based on previously observed actions. For example, an analysis might help determine procedures to follow when new accounts are...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for nitromethane. Justify your answer.
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1, 1, 2-trichloroethane. Justify your answer.
-
Calculate the spin energy eigenvalues for the wave functions 1 = (1) (2), 3 = (1) (2), and 4 = (1) (2). [Equation (28.15)] for noninteracting spins.
-
One of the great issues facing the world is that of growing income inequality. This is a problem with intra-national (within nations) and inter-national (between nations) dynamics. The USA has one of...
-
Simplify a. (64n)3 b. (5b2c4)
-
Evaluate the expression (1+41) (-4 + 21) and write the result in the form a + bi, where is the real part of your answer and is the coefficient in front of the imaginary part.

Study smarter with the SolutionInn App


