Calculate H rxn for the reaction: Use the following reactions and given Hs: FeO3(s) + 3 CO(g)
Question:
Calculate ΔHrxn for the reaction:![]()
Use the following reactions and given ΔH’s: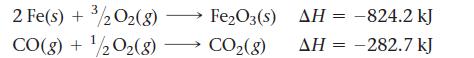
Transcribed Image Text:
Fe₂O3(s) + 3 CO(g) 2 Fe(s) + 3 CO2(8)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A builder gets a construction loan of 10 million. For simplicity, assume the loan has an interest rate at j1=6%. The loan does not require payment during the construction process. Construction will...
-
What are the advantages and disadvantages of organizing a business as a corporation? 2. How does a partnership differ from a limited liability company? 3. Why do corporates file for bankruptcy? 4....
-
Calculate S values for the following reactions by using tabulated values from Appendix C. In each case explain the sign of S. (c) HNO3(g) NH3 (g)- NH4NO3(s) 2 Fe203(s)4 Fe(s) 302(g) CaCO3(s,calcite)...
-
Elm Corp. makes two products: C and D. The following data have been summarized: Indirect manufacturing cost information includes the following: The company plans to manufacture 250 units of each...
-
Juan Ortiz is unable to reconcile the bank balance at January 31. Juans reconciliation is shown here. Cash balance per bank ..... $3,660.20 Add: NSF check ....... 470.00 Less: Bank service charge...
-
State the possible boundary conditions at the ends of a string.
-
Describe how an auditor searches for unrecorded liabilities.
-
Hanson Company (see BE10-2) borrowed $1,000,000 on March 1 on a 5-year, 12% note to help finance construction of the building. In addition, the company had outstanding all year a 10%, 5-year,...
-
where company shows it uneanedd revuene and how it is decribedd in the footnotes?
-
BJ's Wholesale Corporation operates more than 590 membership warehouses and employs more than 164,000 people. Its annual report included the following items at August 31, 2011 (in millions of...
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: CaO(s) + CO2(g) CaCO3(s)
-
Calculate H rxn for the reaction: Use the following reactions and given Hs: 5 C(s) + 6 H(8) C5H12(1)
-
Find Rab in the circuit shown. 2 kQ 2 kQ 2 kQ 2 kQ 4 kQ 4 kQ RAB 2 kQ 2 kQ 2 kQ 2 kQ
-
what ways does inspiration intersect with motivation and drive, propelling individuals towards extraordinary feats of accomplishment ? Explain
-
How does inspiration catalyze the process of creativity, particularly in the realms of art and innovation ?
-
Elements of the Marketing sector in relation to the Real Estate industry in Marbella. Discuss the size of the sector, its economic importance to the industry in Marbella, number of people employed,...
-
Wallace Driving School's 2020 balance sheet showed net fixed assets of $4.7 million, and the 2021 balance sheet showed net fixed assets of $5.3 million. The company's 2021 income statement showed a...
-
2. Evaluate the following integrals. sechr tanha da (a)/sech e2* sinh 2r dr (b) s () 1 dr 2(x-1)2+3 (3 marks) (3 marks) (5 marks)
-
Design the accounting approach of the future. What should accounting in fifteen years look like, to make a maximum contribution to sustainable development, stakeholder value, and moral excellence?
-
What services are provided by the provincial and territorial governments?
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for 1, 1, 2, 2-tetrachloroethane assuming that there is no rotation of the two...
-
Predict the number of chemically shifted 1 H peaks and the multiplet splitting of each peak that you would observe for nitroethane. Justify your answer.
-
The nuclear spin operators can be represented as 2 à 2 matrices and α and β can be represented as column vectors in the form Given that And Show that And IÌ z...
-
In an arcade video game, a spot is programmed to move across the screen according to x = 9.04 t - 0.637 t 3 , where x is the distance in centimeters measured from the left edge of the screen and t is...
-
XYZ Manufacturing Company produces two types of products: Product A and Product B. The company is deciding whether to continue manufacturing Product A or discontinue it. The following information is...
-
A bird has a mass of 26.0 g and perches in the middle of a stretched telephone line. (a) Show that the tension in the line can be calculated using the equation T = (b) Determine the tension when 0 =...

Study smarter with the SolutionInn App


