Calculate the vapor pressure at 25 C of a solution containing 99.5 g sucrose (C 12 H
Question:
Calculate the vapor pressure at 25 °C of a solution containing 99.5 g sucrose (C12H22O11) and 300.0 mL water.
The vapor pressure of pure water at 25 °C is 23.8 torr. Assume the density of water is 1.00 g/mL.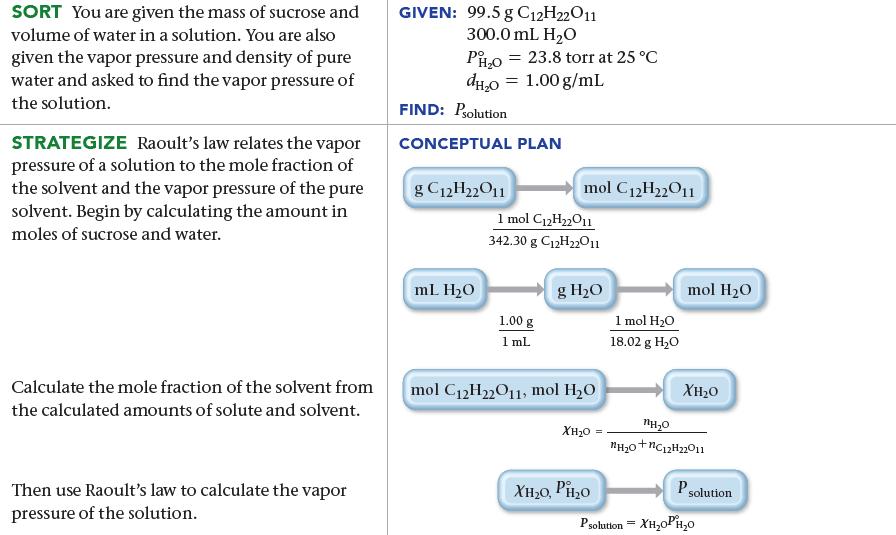
Transcribed Image Text:
SORT You are given the mass of sucrose and volume of water in a solution. You are also given the vapor pressure and density of pure water and asked to find the vapor pressure of the solution. STRATEGIZE Raoult's law relates the vapor pressure of a solution to the mole fraction of the solvent and the vapor pressure of the pure solvent. Begin by calculating the amount in moles of sucrose and water. Calculate the mole fraction of the solvent from the calculated amounts of solute and solvent. Then use Raoult's law to calculate the vapor pressure of the solution. GIVEN: 99.5 g C12H22O11 300.0 mL H₂O PH₂O dH₂0 FIND: Psolution CONCEPTUAL PLAN g C12H22011 mL H₂O = 23.8 torr at 25 °C 1.00 g/mL mol C12H22011 1 mol C₁2H₂2011 342.30 g C12H22011 1.00 g 1 mL g H₂O mol C12H22011, mol H₂O XH₂0 XH,O, PH,o 1 mol H₂O 18.02 g H₂O mol H₂O XH₂O PHO *Hạo+nC_zHzzO11 P solution Psolution = XH₂OPH₂0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
995 g C12H22O11 X 3000 mL HO x XHO nHO nC12H2...View the full answer

Answered By

Allan Simiyu
I am an adroit Writer. I am a dedicated writer having worked as a writer for 3 years now. With this, I am sure to ace in the field by helping students break down abstract concepts into simpler ideas.
5.00+
8+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the vapor pressure at 25 C of a solution containing 165 g of the nonvolatile solute, glucose, C 6 H 12 O 6 , in 685 g H 2 O. The vapor pressure of water at 25 C is 23.8 mmHg.
-
Determine the vapor pressure at 25 C of an aqueous ethylene glycol (C 2 H 6 O 2 ) solution that is 14.8 % C 2 H 6 O 2 by mass. The vapor pressure of pure water at 25 C is 23.8 torr. a) 3.52 torr b)...
-
Calculate the vapor pressure at 35C of a solution made by dissolving 20.2 g of sucrose, C12H22O11, in 70.1 g of water. The vapor pressure of pure water at 35C is 42.2 mmHg. What is the vapor-pressure...
-
Where is the line in the sand the point where such behaviors are so destructive that you feel that the relationship needs to end?
-
Life-cycle cost reduction is best achieved during the development stage of the production life cycle. Do you agree or disagree? Explain.
-
A sample of two items is selected without replacement from a batch. Describe the (ordered) sample space for each of the following batches: (a) The batch contains the items {a, b, c, d}. (b) The batch...
-
List the key SE principles learned from this chapter.
-
Clean Corporation manufactures liquid window cleaner. The following information concerns its work in process: Beginning inventory, 8,000 partially complete gallons. Transferred out, 42,000 gallons....
-
A tool that migrates data from database A to B has been developed. The tool will be used to migrate all production data from version 1.0 of our app (operating on A) to version 2.0, currently being...
-
A sodium nitrate solution is 12.5% NaNO 3 by mass and has a density of 1.02 g/mL. Calculate the molarity of the solution. a) 1.44 M b) 12.8 M c) 6.67 M d) 1.50 M
-
A solution is saturated in both nitrogen gas and potassium bromide at 75 C. When the solution is cooled to room temperature, what is most likely to happen? (a) Some nitrogen gas bubbles out of...
-
Why do we prefer to use semiconductor crystals that contain as small a number of dislocations as possible?
-
1. How would you write up a follow-up investigation supplemental report on the case below? 2. How would you describe the facts of this case along with the information about the preparator? 3. what...
-
Jamie Lee Jackson, age 27, full-time student and part-time bakery employee, has just moved into a bungalow-style, unfurnished home of her own. The house is only a one-bedroom, but the rent is...
-
Nike had sales of $44.487 billion in 2021. Suppose you expected its sales to grow at a rate of 18% in 2022, but then slow by 3% per year to the long-run growth rate that is characeristic of the...
-
How do you suppose households had monies during COVID-19 to purchase goods and services? Is it possible that our country's aggregate demand curve has shifted to the right and caused our economy to...
-
The Denver Corporation has forecast the following sales for the first seven months of the year: January February $ 20,000 22,000 March 24,000 April 30,000 May 20,000 June July 26,000 28,000 Monthly...
-
Distinguish between the two major methods used to account for revenue under long-term contracts.
-
Explain what is meant by vicarious liability and when it is available?
-
What pressure is required above the water in Fig. 6.13 to cause the jet to rise to 9.50 m? The water depth is 1.50 m. 40.0 ft Air pressure h = 6.0 ft
-
What pressure is required above the water in Fig. 6.12 to cause the jet to rise to 28.0 ft? The water depth is 4.50 ft. h
-
To what height will the jet of water rise for the conditions shown in Fig. 6.40? p= 12.0 psig Jet 3.50 ft 3 in 9 in
-
(h) The table below shows a binary term-document incidence matrix for a sample data collection: Gel 0 Animal 1 Spring Car 1 Window Flower 1 BORHOOD D1 1 0 1 0 0 0 1 PROTOC D2 D3 1 1 1 0 1 0 POHODNO...
-
A person has a quadratic utility function: u(w) = w- Bw2, where > 0 is some constant. (Assume the wealth levels are such that 1 - 2w > 0, so that marginal utility is positive. What is the Arrow-Pratt...
-
A physical inventory of Liverpool Company taken at December 31 reveals the following. Per Unit Item Units Cost Market Car audio equipment Speakers 343 $ 98 $ 106 Stereos 258 119 108 Amplifiers 324 94...

Study smarter with the SolutionInn App


