Carbon monoxide and chlorine gas react to form phosgene (COCl 2 ) according to the equation: The
Question:
Carbon monoxide and chlorine gas react to form phosgene (COCl2) according to the equation:![]()
The rate law for the reaction is Rate = k[Cl2]3/2[CO]. Which representation of a mixture of chlorine gas and carbon monoxide gas has the fastest initial rate?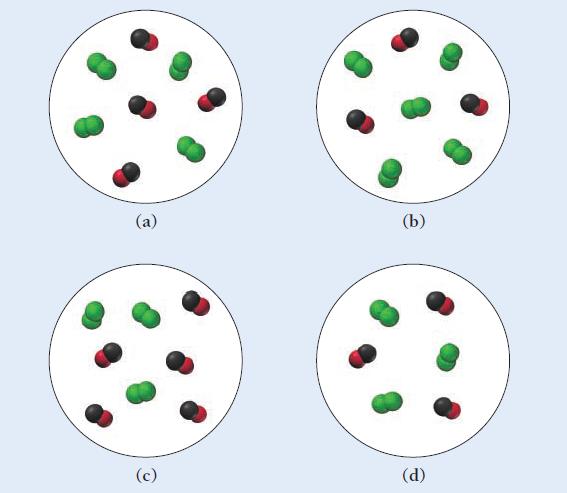
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





