Consider the reaction for the decomposition of hydrogen disulfide: A 0.500-L reaction vessel initially contains 0.0125 mol
Question:
Consider the reaction for the decomposition of hydrogen disulfide: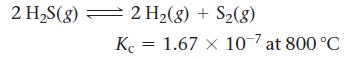
A 0.500-L reaction vessel initially contains 0.0125 mol of H2S at 800 °C. Find the equilibrium concentrations of H2 and S2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





