Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H
Question:
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (ΔH °f for BrCl is 14.6 kJ/mol.)
Problem 74
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction. ΔG°f for BrCl(g) is -1.0 kJ/mol.
![]()
Appendix IIB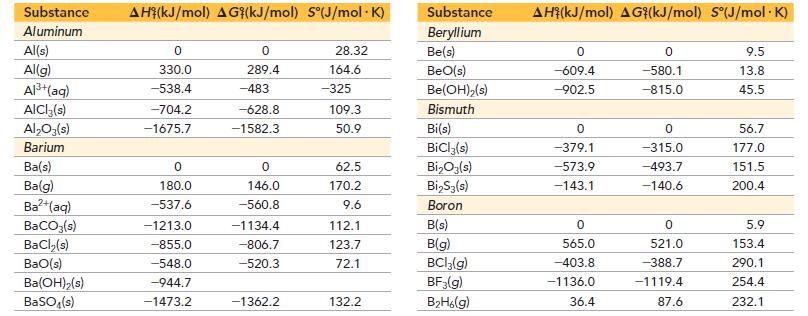
Transcribed Image Text:
a. 2 NO₂(g) N₂O4(8) b. Br₂(g) + Cl₂(g) = 2 BrCI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
To estimate the equilibrium constant K at 655 K for the reaction involving BrCl we can use the van t Hoff equation which relates the change in the equ...View the full answer

Answered By

Gabriela Rosalía Castro
I have worked with very different types of students, from little kids to bussines men and women. I have thaught at universities, schools, but mostly in private sessions for specialized purpuses. Sometimes I tutored kids that needed help with their classes at school, some others were high school or college students that needed to prepare for an exam to study abroud. Currently I'm teaching bussiness English for people in bussiness positions that want to improve their skills, and preparing and ex-student to pass a standarized test to study in the UK.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Compute the determinants by cofactor expansions. At each step, choose a row or column that involves the least amount of computation. 6 9 824 8 -5 0 2 0 2 0-4 6 0 0 3 2 4 0 1 0 7 1 0 0
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G f for BrCl(g) is -1.0 kJ/mol. Appendix IIB a. 2 NO(g) NO4(8) b. Br(g) + Cl(g) = 2 BrCI(g)
-
Do laws provide a complete guide to ethical behavior? Can an activity be legal but not ethical?
-
Which of the following products would typically be accounted for using a job order costing system? Which would typically be accounted for using a process costing system? (a) Paint, (b) Jelly beans,...
-
Why is an accounting system with adequate controls a legal requirement for companies that sell shares of stock to the public?
-
In the benzene adsorber of Example 9.7, the flow rate is increased to \(0.25 \mathrm{~m}^{3} / \mathrm{s}\). Calculate the breakthrough time and the fraction of the bed adsorption capacity that has...
-
Vertical analysis (common size) percentages for Kochheim Companys sales revenue, cost of goods sold, and expenses are shown below. Did Kochheims net income as a percentage of sales increase,...
-
According to the Civil Code of the Philippines, Article 1 7 6 7 defines partnership as: By the contract of partnership two or more persons bind themselves to contribute money, property, or industry...
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
Which process results in the increase in entropy of the universe? a) The cooling of a hot cup of coffee in room temperature air b) The evaporation of water from a desk at room temperature c) The...
-
Which psychographic and behavioristic variables does MBSC use, and why?
-
You throw a ball from your window 8.0 m above the ground. When the ball leaves your hand, it is moving at 12m/s at an angle of 20 0 below the horizontal. Solve for the range.
-
the media literacy section talks about the magazine Cosmopolitan and its focus on using "thin cover models as aspirational objects for its readers -- that is, as women its readers would like to look...
-
Air at 298 K,55% saturated with water vapor is initially at 100 kPa. it is then compressed to 1000 kpa and cooled to a temperature so that 90% of the water vapor is condensed. calculate the final...
-
Improving Cash Flow through Receivable Management Star Communications designs, manufactures, and sells telecommunication equipment, and provides services associated with their installation,...
-
Is Power everything during a negotiation? Make a situation where the party with less power may win the negotiation process. You have to take into consideration the sources of power and the types of...
-
1. Using Microsoft Excel, draw a graph illustrating the supply and demand in this market. 2. What is the equilibrium Price and Quantity in the market? 3. Now suppose the government imposes a special...
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
A steel cylinder of length 11 cm and radius R = 3.5 cm (Fig. P11.53A) is subjected to a compressive force of 5000 N. By what amount??L is the cylinder compressed? Figure P11.53A ? R F F -L
-
Youngs modulus for bone under compression is about 9 10 9 Pa, and the breaking stress is about 2 10 8 Pa. (a) A leg bone of length 30 cm when unstressed is stressed to its breaking point. What is...
-
A guitar string of diameter 0.50 mm and length 0.75 m is subject to a tension of 150 N. If the string stretches an amount 0.40 mm, what is Youngs modulus of the string?
-
In your company ( or in an e Commerce retailer that you purchase from ), recommend how you ( they ) should conduct an ABC Analysis and a XYZ Analysis. Evaluate if this is currently being done...
-
Create a company blog for PUMA proforma income balance sheets and comment on the process of creating the proforma documents as well as your conclusions on the need for external financing needs with...
-
You will research the following about Bill Gates: Professional career track Management and leadership styles Team building skills Organizational vision and culture Problem-solving and conflict...

Study smarter with the SolutionInn App


