Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. G
Question:
Use data from Appendix IIB to calculate the equilibrium constants at 25 °C for each reaction. ΔG°f for BrCl(g) is -1.0 kJ/mol.![]()
Appendix IIB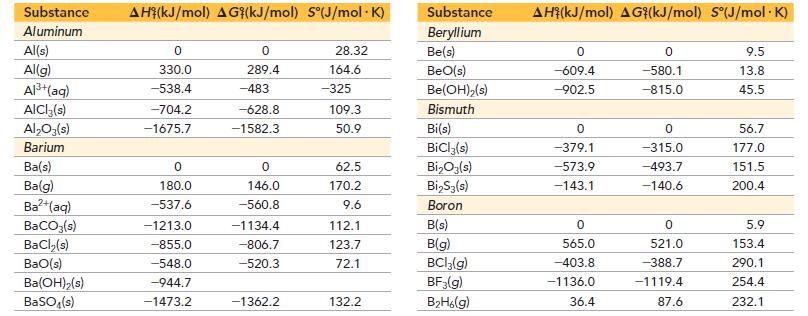
Transcribed Image Text:
a. 2 NO₂(g) N₂O4(8) b. Br₂(g) + Cl₂(g) = 2 BrCI(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the equilibrium constant K at 25 C for a given reaction from standard Gibbs free energy of formation Gf values you can use the following ...View the full answer

Answered By

Mahesh G
I have more than 7 years of experience in teaching physics, mathematics and python programming to more than 600 students including both online and offline tutoring.
I follow the following 7 step fundamental approach towards tutoring.
1. Curiosity, scope, enlightenment of the topic in hand.
2. Problem Definitions and elaboration.
3. Requisite mathematics, analytical abilities and quantitative
aptitude.
4. Preparing Algorithms for problem statement.
5. Concepts with analogies and building algorithm.
6. Introspection and improvising.
7. Daily class wise Cheat sheets(its not cheating) for consolidation.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use data from Appendix IIB to calculate the equilibrium constants at 25 C for each reaction. Appendix IIB a. 2 CO(g) + O(g) = 2 CO(g) b. 2 HS(g) = 2 H(g) + S(g)
-
Estimate the value of the equilibrium constant at 655 K for each reaction in Problem 74. (H f for BrCl is 14.6 kJ/mol.) Problem 74 Use data from Appendix IIB to calculate the equilibrium constants at...
-
Use data from Appendix C to calculate the equilibrium constant, K, at 298 K for each of the following reactions: H2(g) + 12(g) 2 HI(g) C2H5OH (g)- C2H4(g) + H2O(g)
-
1. A projectile is launched in a vertical plane, at an angle 0 with initial velocity vo. It must be caught in a frictionless circular tube of radius R in such a way that the trajectory of the...
-
Bell Printing Company specializes in wedding in wedding invitations. Bell needs information to budget next years activities. Write yes or no to indicate whether each of the following costs is likely...
-
Tasaka Company manufactures oriental rugs. It pays utility bills at the end of the month in which services are received. The company received the following bills for April, May, and June,...
-
Repeat the calculations of Example 9.5, but for a total solution normality of 0.5. Data From Example 9.5:- For the Cu 2+ /Na + exchange with a strong-acid resin, show how the fraction CuR2 in the...
-
(2-Year Worksheet) On January 1, 2010, Cunningham Company has the following defined benefit pension plan balances.? Projected benefit obligation ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ?$4,500,000 Fair...
-
Calculate inventory amounts when costs are declining During the year, Hooker Incorporated has the following inventory transactions. Date January 1 Transaction Beginning inventory March 4 Purchase...
-
Consider the reaction: Calculate G rxn for the reaction at 25 C under each of the following conditions: a. Standard conditions b. At equilibrium c. P CH3OH = 1.0 atm; P CO = P H2 = 0.010 atm CO(g) +...
-
Which process results in the increase in entropy of the universe? a) The cooling of a hot cup of coffee in room temperature air b) The evaporation of water from a desk at room temperature c) The...
-
Develop an investment opportunity schedule (IOS) for ProChem.
-
Maya is using some rope to pull a sled up a snow-covered hill. Friction between the sled and the hill is negligible. The vertical distance between the bottom and the top of the hill is h = 17 m. The...
-
a) A business has semi-variable overhead costs associated with its manufacturing output. It wants to be able to better plan and control its production, and therefore needs determine which of these...
-
(b) Gross pay 10,30,000 (including cost of idle time hours paid to employee *25,000); Accommodation provided to employee free of cost [this accommodation is owned by employer, depreciation of...
-
Can Kruskal's algorithm be adapted to find (a) a maximum-weight tree in a weighted connected graph? (b) a minimum-weight maximal forest in a weighted graph? If so, how?
-
You are a senior auditor working on the audit of HealthyGlow for the year ended 30 June 2021. You are in the planning stage of the audit. It is April 2021 and you discover that HealthyGlow has...
-
Define the different types of unemployment
-
An 8.0 kg crate is pulled 5.0 m up a 30 incline by a rope angled 18 above the incline. The tension in the rope is 120 N, and the crates coefficient of kinetic friction on the incline is 0.25. a. How...
-
If the frequency of a wave is doubled, how does the wavelength change?
-
A solid glass sphere with an initial diameter d = 25.00 cm is subject to a pressure of 1000 P atm , where atmospheric pressure P atm = 1.01 10 5 Pa. What is the new diameter of the sphere? The...
-
A shear force of 3.0 ? 10 5 N is applied to an aluminum bar of length L = 20 cm, width w = 5.0 cm, and height h = 2.0 cm as shown in Figure 11.23 . What is the shear deformation??x? Figure 11.23 ? A...
-
Discussing how to build a supply base. How to improve the port of LA and Los Angeles to make it more efficient. He said that the port of Singapore is the most effective port.
-
Use at minimum the following theories discussed in this competency:Organizational Capacity for Change Accountable Culturesand the Organizational Capacity for Change Innovative Cultures.Use all of the...
-
Ethically speaking, what groups of stakeholders does management have to consider in case of a disaster? a priority list of the stakeholders of the Indiana State Fair organization and why each group...

Study smarter with the SolutionInn App


