Find the amount (in moles) of CC bonds that must be broken when 1.0 mole of C(g)
Question:
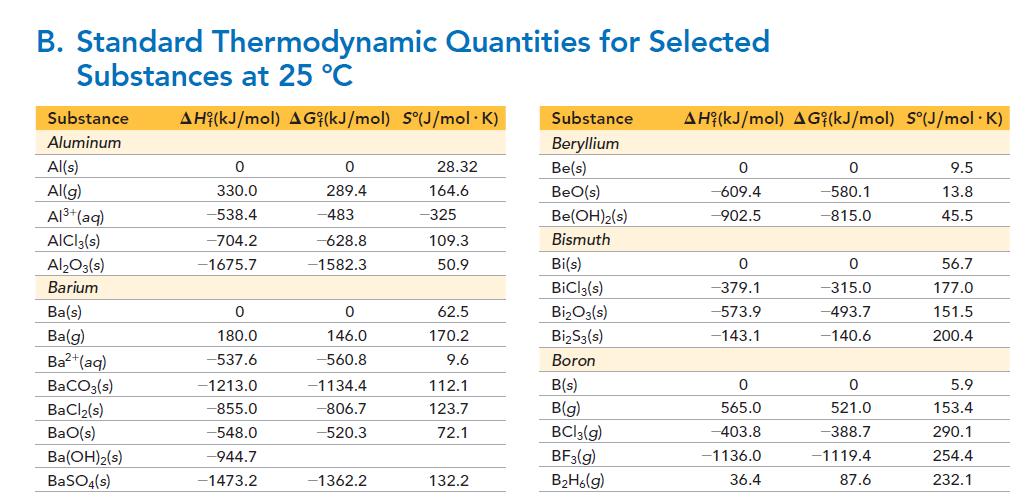
Find the amount (in moles) of C—C bonds that must be broken when 1.0 mole of C(g) is formed from C(diamond). Calculate the ΔH of sublimation of diamond from the data in Appendix II, Table B. Then do the calculation using the C—C bond energy in Table 10.3. Suggest a reason for the difference between the two values.
Transcribed Image Text:
TABLE 10.3 Average Bond Energies Bond Energy (kJ/mol) 436 414 Bond H-H H-C H-N H-O H-S H-F H-CI H-Br H-I C-C C C C=C C-N C-N C=N C-O CIO C=O C-CI *799 in CO₂ 389 464 368 565 431 364 297 347 611 837 305 615 891 360 736* 1072 339 Bond N-N N=N N=N N-O N=O N-F N-CI N-Br N-I 0-0 0-0 O-F O-CI 0-1 F-F CI-F CI-CI Bond Energy (kJ/mol) 163 418 946 222 590 272 200 243 159 142 498 190 203 234 159 253 243 Bond Br-F Br-Cl Br-Br I-CI 1-Br 1-1 Si-H Si-Si Si-C S-O Si-O S=O Si-Cl S-S S-F S-CI S-Br S-S Bond Energy (kJ/mol) 237 218 193 208 175 151 323 226 301 265 368 523 464 418 327 253 218 266
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
20 mol of CC bonds 715 kJmol 69 10 2 kJ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-6. On December 12, Irene purchased the building where her store is located. She paid...
-
McGuire Industries prepares budgets to help manage the company. McGuire is budgeting for the fiscal year ended January 31, 2021. During the preceding year ended January 31, 2020, sales totaled $9,200...
-
Refer to the statement of stockholders equity for Crisanti Corporation in Exhibit 14-4 to answer the following questions: (1) At what price per share were the 10,000 shares of common stock sold? (2)...
-
Epstein Company, a wholesale distributor of jewelry, sells to retail jewelry stores on terms of net 120. Its average collection period is 150 days. The company is considering the introduction of a 4...
-
Refer to Samsungs financial statements in Appendix A. Compute its debt ratio as of December 31, 2015, and December 31, 2014. Data From Samsung Financial Statement Appendix A Samsung Electronics Co.,...
-
Lon Timur is an accounting major at a midwestern state university located approximately 60 miles from a major city. Many of the students attending the university are from the metropolitan area and...
-
Suppose your yearly demand for razors is Q = 32 - 4P. There is a subscription service that charges $2.00 per razor plus an annual membership fee. What is the most that you would be willing to pay for...
-
Using the molecular orbital model for a diatomic molecule, explain the different bond lengths for the ions of oxygen. Also state which ion is diamagnetic. lon O+ 0 0 0- -O Bond Length (pm) 112 121...
-
Sodium peroxide is a very powerful oxidizing agent. Balance the reaction of sodium peroxide with elemental iron to give sodium oxide and Fe 3 O 4 .
-
In what ways are the maintenance and repair decision and the rehabilitation decision similar? How do they differ?
-
In today's fast-paced global markets, we are often interested in distributing leadership throughout the organization appropriately, empowering each employee to step forward to share his or her...
-
Make a recommend for Market Entry Strategy to Automative Industry (Tesla) for selected BRIC country China, after examining the pros and cons of each possible strategy in the table below. Discuss...
-
VWoA (Volkswagen of America) uses an OCI (Option Creation Category) when they categorize their projects. What do they mean by this and why would they use this category? How does IVK align Business...
-
You work for an energy utility company that provides heating and cooling services (HVAC) to residential clients in Ontario. The company is trying to increase its awareness and build clients in the...
-
1.Questions of purpose. What are your personal and professional goals? What do you hope to accomplish? What would make your professional life worthwhile? 2. Questions of risk. What is your risk...
-
Discuss the Gunpowder revolution and the changes that took place in technology, architecture, and science etc...? What was overall impact of Gunpowder weapons on World Societies?
-
SCHEDULE OF COST OF GOODS MANUFACTURED The following information is supplied for Sanchez Welding and Manufacturing Company. Prepare a schedule of cost of goods manufactured for the year ended...
-
A ball of positive charge rotates about a vertical axis (Fig. P20.61). (a) If the rotation is clockwise as viewed from above, what is the direction of the balls magnetic moment? (b) If the charge on...
-
. Velocity selector. Consider a charged particle moving through a region in which the electric field is perpendicular to the magnetic field, with both fields perpendicular to the initial velocity of...
-
You wish to design a velocity selector (see Problem 62 and Fig. P20.62) that will allow protons to pass through only if they have a speed of 500 m/s. (a) If the magnetic field is B = 0.050 T, what...
-
1) Predict the product(s) of the following reactions. Is it SN1, SN2, E1, or E2 mechanism? CH3 a) b) Br (i-Pr)2N THF d) CH3 Br NC THF N3 Na MeOH c) Br MeOH, heat Br t-BuO Nal CI THF Acetone 2)...
-
1. (15 pts) Do both swap_functions work? If not, fix the one(s) that does not work. #include void swap_nums (int *x, int *y) { int tmp; tmp = *x; *x = *y; *y = tmp; } void swap pointers (char *x,...
-
1) Develop well-documented pseudo code that finds all the elements of a given array (of any size n) that are multiple of x. The code must display the indices and the values of these elements. For...

Study smarter with the SolutionInn App


